Development of a programmed cell death ligand-1 immunohistochemical assay validated for analysis of non-small cell lung cancer and head and neck squamous cell carcinoma
- PMID: 27717372
- PMCID: PMC5055695
- DOI: 10.1186/s13000-016-0545-8
Development of a programmed cell death ligand-1 immunohistochemical assay validated for analysis of non-small cell lung cancer and head and neck squamous cell carcinoma
Abstract
Background: A high-quality programmed cell-death ligand 1 (PD-L1) diagnostic assay may help predict which patients are more likely to respond to anti-programmed cell death-1 (PD-1)/PD-L1 antibody-based cancer therapy. Here we describe a PD-L1 immunohistochemical (IHC) staining protocol developed by Ventana Medical Systems Inc. and key analytical parameters of its use in formalin-fixed, paraffin-embedded (FFPE) samples of non-small cell lung cancer (NSCLC) and head and neck squamous cell carcinoma (HNSCC).
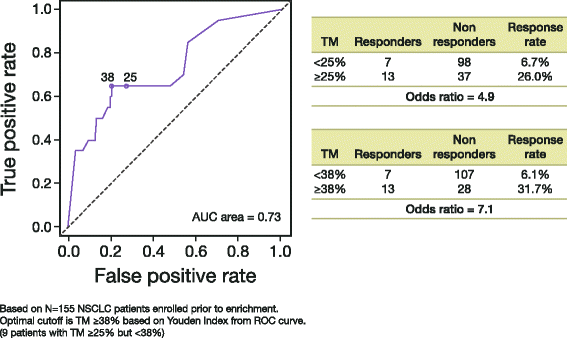
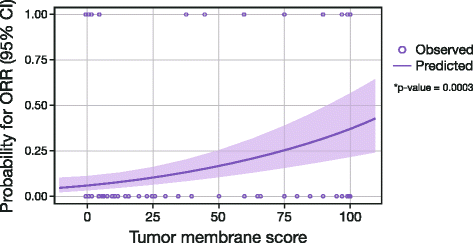
Methods: An anti-human PD-L1 rabbit monoclonal antibody (SP263) was optimized for use with the VENTANA OptiView DAB IHC Detection Kit on the automated VENTANA BenchMark ULTRA platform. The VENTANA PD-L1 (SP263) Assay was validated for use with FFPE NSCLC and HNSCC tissue samples in a series of studies addressing sensitivity, specificity, robustness, and precision. Samples from a subset of 181 patients from a Phase 1/2 study of durvalumab (NCT01693562) were analyzed to determine the optimal PD-L1 staining cut-off for enriching the probability of responses to treatment. The scoring algorithm was defined using statistical analysis of clinical response data from this clinical trial and PD-L1 staining parameters in HNSCC and NSCLC tissue. Inter-reader agreement was established by three pathologists who evaluated 81 NSCLC and 100 HNSCC samples across the range of PD-L1 expression levels.
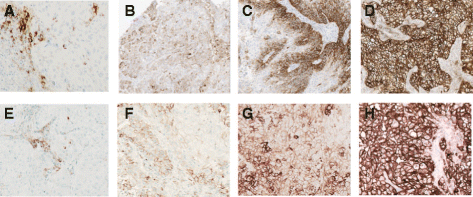
Results: The VENTANA PD-L1 (SP263) Assay met all pre-defined acceptance criteria. For both cancer types, a cut-off of 25 % of tumor cells with PD-L1 membrane staining of any intensity best discriminated responders from nonresponders. Samples with staining above this value were deemed to have high PD-L1 expression, and those with staining below it were deemed to have low or no PD-L1 expression. Inter-reader agreement on PD-L1 status was 97 and 92 % for NSCLC and HNSCC, respectively.
Conclusions: These results highlight the robustness and reproducibility of the VENTANA PD-L1 (SP263) Assay and support its suitability for use in the evaluation of NSCLC and HNSCC FFPE tumor samples using the devised ≥25 % tumor cell staining cut-off in a clinical setting. The clinical utility of the PD-L1 diagnostic assay as a predictive biomarker will be further validated in ongoing durvalumab studies.
Trial registration: ClinicalTrials.gov: NCT01693562.
Keywords: Assay; Diagnostic; Durvalumab; HNSCC; Immunohistochemistry; Immunotherapy; MEDI4736; NSCLC; PD-L1.
Figures
Similar articles
-
Consistency of tumor and immune cell programmed cell death ligand-1 expression within and between tumor blocks using the VENTANA SP263 assay.Diagn Pathol. 2018 Jul 24;13(1):47. doi: 10.1186/s13000-018-0725-9. Diagn Pathol. 2018. PMID: 30041679 Free PMC article.
-
Analytical Validation and Clinical Utility of an Immunohistochemical Programmed Death Ligand-1 Diagnostic Assay and Combined Tumor and Immune Cell Scoring Algorithm for Durvalumab in Urothelial Carcinoma.Arch Pathol Lab Med. 2019 Jun;143(6):722-731. doi: 10.5858/arpa.2017-0555-OA. Epub 2018 Nov 20. Arch Pathol Lab Med. 2019. PMID: 30457897 Clinical Trial.
-
Automated image analysis of NSCLC biopsies to predict response to anti-PD-L1 therapy.J Immunother Cancer. 2019 May 6;7(1):121. doi: 10.1186/s40425-019-0589-x. J Immunother Cancer. 2019. PMID: 31060602 Free PMC article. Clinical Trial.
-
Programmed Death-Ligand 1 Immunohistochemistry Testing: A Review of Analytical Assays and Clinical Implementation in Non-Small-Cell Lung Cancer.J Clin Oncol. 2017 Dec 1;35(34):3867-3876. doi: 10.1200/JCO.2017.74.7642. Epub 2017 Oct 20. J Clin Oncol. 2017. PMID: 29053400 Review.
-
[Predictive PD-L1 immunohistochemistry for non-small cell lung cancer : Current state of the art and experiences of the first German harmonization study].Pathologe. 2016 Nov;37(6):557-567. doi: 10.1007/s00292-016-0189-1. Pathologe. 2016. PMID: 27510417 Review. German.
Cited by
-
Durvalumab With or Without Tremelimumab in Combination With Chemotherapy as First-Line Therapy for Metastatic Non-Small-Cell Lung Cancer: The Phase III POSEIDON Study.J Clin Oncol. 2023 Feb 20;41(6):1213-1227. doi: 10.1200/JCO.22.00975. Epub 2022 Nov 3. J Clin Oncol. 2023. PMID: 36327426 Free PMC article. Clinical Trial.
-
Concordance of Programmed Death-Ligand 1 Expression between SP142 and 22C3/SP263 Assays in Triple-Negative Breast Cancer.J Breast Cancer. 2020 Jun;23(3):303-313. doi: 10.4048/jbc.2020.23.e37. J Breast Cancer. 2020. PMID: 32595992 Free PMC article.
-
The prognostic value of PD-L1 expression in upper tract urothelial carcinoma varies according to platelet count.Cancer Med. 2018 Sep;7(9):4330-4338. doi: 10.1002/cam4.1686. Epub 2018 Jul 31. Cancer Med. 2018. PMID: 30062756 Free PMC article.
-
Four immunohistochemical assays to measure the PD-L1 expression in malignant pleural mesothelioma.Oncotarget. 2018 Apr 17;9(29):20769-20780. doi: 10.18632/oncotarget.25100. eCollection 2018 Apr 17. Oncotarget. 2018. PMID: 29755688 Free PMC article.
-
Immunohistochemical comparison of three programmed death-ligand 1 (PD-L1) assays in triple-negative breast cancer.PLoS One. 2021 Sep 24;16(9):e0257860. doi: 10.1371/journal.pone.0257860. eCollection 2021. PLoS One. 2021. PMID: 34559865 Free PMC article.
References
-
- Rebelatto M, Mistry A, Sabalos C, Walker J, Midha A, Steele K, et al. Development of a PD-L1 Companion Diagnostic Assay for Treatment With MEDI4736 in NSCLC and SCCHN Patients. Chicago: Poster presented at the 51st American Society of Clinical Oncology Annual Meeting; 2015.
-
- Rebelatto MC, Mistry A, Sabalos C, Schechter N, Walker J, Midha A, et al. An immunohistochemical PD-L1 companion diagnostic assay for treatment with durvalumab (MEDI4736) in NSCLC patients. Cancer Immunol Res. 2016;4(1 Suppl):B005. doi: 10.1158/2326-6074.CRICIMTEATIAACR15-B005. - DOI
-
- D’Addario G, Früh M, Reck M, Baumann P, Klepetko W, Felip E. Metastatic non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(5_Suppl):v116–9. doi:10.1093/annonc/mdq189. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

