NSAID-activated gene 1 mediates pro-inflammatory signaling activation and paclitaxel chemoresistance in type I human epithelial ovarian cancer stem-like cells
- PMID: 27708225
- PMCID: PMC5342151
- DOI: 10.18632/oncotarget.12355
NSAID-activated gene 1 mediates pro-inflammatory signaling activation and paclitaxel chemoresistance in type I human epithelial ovarian cancer stem-like cells
Abstract
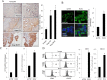
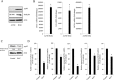
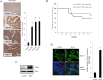
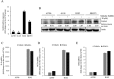
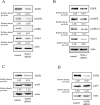
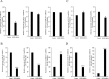
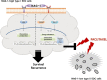
Epithelial ovarian cancer (EOC) remains the most lethal gynecologic malignancy in developed countries. Chronic endogenous sterile pro-inflammatory responses are strongly linked to EOC progression and chemoresistance to anti-cancer therapeutics. In the present study, the activity of epithelial NF-κB, a key pro-inflammatory transcription factor, was enhanced with the progress of EOC. This result was mechanistically linked with an increased expression of NSAID-Activated Gene 1 (NAG-1) in MyD88-positive type I EOC stem-like cells, compared with that in MyD88-negative type II EOC cells. Elevated NAG-1 as a potent biomarker of poor prognosis in the ovarian cancer was positively associated with the levels of NF-κB activation, chemokines and stemness markers in type I EOC cells. In terms of signal transduction, NAG-1-activated SMAD-linked and non-canonical TGFβ-activated kinase 1 (TAK-1)-activated pathways contributed to NF-κB activation and the subsequent induction of some chemokines and cancer stemness markers. In addition to effects on NF-κB-dependent gene regulation, NAG-1 was involved in expression of EGF receptor and subsequent activation of EGF receptor-linked signaling. The present study also provided evidences for links between NAG-1-linked signaling and chemoresistance in ovarian cancer cells. NAG-1 and pro-inflammatory NF-κB were positively associated with resistance to paclitaxel in MyD88-positive type I EOC cells. Mechanistically, this chemoresistance occurred due to enhanced activation of the SMAD-4- and non-SMAD-TAK-1-linked pathways. All of the present data suggested NAG-1 protein as a crucial mediator of EOC progression and resistance to the standard first-line chemotherapy against EOC, particularly in MyD88-positive ovarian cancer stem-like cells.
Keywords: NAG-1; NF-κB; chemoresistance; epithelial ovary cancer; paclitaxel.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures




Similar articles
-
Differential activation of NF-κB signaling is associated with platinum and taxane resistance in MyD88 deficient epithelial ovarian cancer cells.Int J Biochem Cell Biol. 2015 Apr;61:90-102. doi: 10.1016/j.biocel.2015.02.001. Epub 2015 Feb 11. Int J Biochem Cell Biol. 2015. PMID: 25681684
-
Atractylenolide-I sensitizes human ovarian cancer cells to paclitaxel by blocking activation of TLR4/MyD88-dependent pathway.Sci Rep. 2014 Jan 23;4:3840. doi: 10.1038/srep03840. Sci Rep. 2014. PMID: 24452475 Free PMC article.
-
Phenotypic modifications in ovarian cancer stem cells following Paclitaxel treatment.Cancer Med. 2013 Dec;2(6):751-62. doi: 10.1002/cam4.115. Epub 2013 Aug 27. Cancer Med. 2013. PMID: 24403249 Free PMC article.
-
Advances in the treatment of ovarian cancer: a potential role of antiinflammatory phytochemicals.Discov Med. 2012 Jan;13(68):7-17. Discov Med. 2012. PMID: 22284780 Review.
-
Cancerous ovarian stem cells: obscure targets for therapy but relevant to chemoresistance.J Cell Biochem. 2013 Jan;114(1):21-34. doi: 10.1002/jcb.24317. J Cell Biochem. 2013. PMID: 22887554 Review.
Cited by
-
Stress-responsive Gdf15 counteracts renointestinal toxicity via autophagic and microbiota reprogramming.Commun Biol. 2023 Jun 3;6(1):602. doi: 10.1038/s42003-023-04965-1. Commun Biol. 2023. PMID: 37270567 Free PMC article.
-
Enterocyte-Based Bioassay via Quantitative Combination of Proinflammatory Sentinels Specific to 8-keto-trichothecenes.Front Immunol. 2020 Jul 16;11:1530. doi: 10.3389/fimmu.2020.01530. eCollection 2020. Front Immunol. 2020. PMID: 32765531 Free PMC article.
-
Growth differentiation factor 15 induces cisplatin resistance through upregulation of xCT expression and glutathione synthesis in gastric cancer.Cancer Sci. 2023 Aug;114(8):3301-3317. doi: 10.1111/cas.15869. Epub 2023 Jun 1. Cancer Sci. 2023. PMID: 37260027 Free PMC article.
-
GPX3 supports ovarian cancer tumor progression in vivo and promotes expression of GDF15.Gynecol Oncol. 2024 Jun;185:8-16. doi: 10.1016/j.ygyno.2024.02.004. Epub 2024 Feb 10. Gynecol Oncol. 2024. PMID: 38342006
-
GDF15 promotes glioma stem cell-like phenotype via regulation of ERK1/2-c-Fos-LIF signaling.Cell Death Discov. 2021 Jan 11;7(1):3. doi: 10.1038/s41420-020-00395-8. Cell Death Discov. 2021. PMID: 33431816 Free PMC article.
References
-
- Murdoch WJ, McDonnel AC. Roles of the ovarian surface epithelium in ovulation and carcinogenesis. Reproduction. 2002;123:743–750. - PubMed
-
- Schwartz PE. Current diagnosis and treatment modalities for ovarian cancer. Cancer Treat Res. 2002;107:99–118. - PubMed
-
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. - PubMed
-
- Lu KH, Patterson AP, Wang L, Marquez RT, Atkinson EN, Baggerly KA, Ramoth LR, Rosen DG, Liu J, Hellstrom I, Smith D, Hartmann L, Fishman D, et al. Selection of potential markers for epithelial ovarian cancer with gene expression arrays and recursive descent partition analysis. Clin Cancer Res. 2004;10:3291–3300. - PubMed
-
- Daly M, Obrams GI. Epidemiology and risk assessment for ovarian cancer. Semin Oncol. 1998;25:255–264. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

