Foot-and-Mouth Disease Virus Viroporin 2B Antagonizes RIG-I-Mediated Antiviral Effects by Inhibition of Its Protein Expression
- PMID: 27707918
- PMCID: PMC5126369
- DOI: 10.1128/JVI.01310-16
Foot-and-Mouth Disease Virus Viroporin 2B Antagonizes RIG-I-Mediated Antiviral Effects by Inhibition of Its Protein Expression
Erratum in
-
Erratum for Zhu et al., "Foot-and-Mouth Disease Virus Viroporin 2B Antagonizes RIG-I-Mediated Antiviral Effects by Inhibition of Its Protein Expression".J Virol. 2021 Jun 10;95(13):e0054521. doi: 10.1128/JVI.00545-21. Epub 2021 Jun 10. J Virol. 2021. PMID: 34110266 Free PMC article. No abstract available.
Abstract
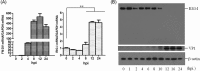
The role of retinoic acid-inducible gene I (RIG-I) in foot-and-mouth disease virus (FMDV)-infected cells remains unknown. Here, we showed that RIG-I inhibits FMDV replication in host cells. FMDV infection increased the transcription of RIG-I, while it decreased RIG-I protein expression. A detailed analysis revealed that FMDV leader proteinase (Lpro), as well as 3C proteinase (3Cpro) and 2B protein, decreased RIG-I protein expression. Lpro and 3Cpro are viral proteinases that can cleave various host proteins and are responsible for several of the viral polyprotein cleavages. However, for the first time, we observed 2B-induced reduction of host protein. Further studies showed that 2B-mediated reduction of RIG-I is specific to FMDV, but not other picornaviruses, including encephalomyocarditis virus, enterovirus 71, and coxsackievirus A16. Moreover, we found the decreased protein level of RIG-I is independent of the cleavage of eukaryotic translation initiation factor 4 gamma, the induction of cellular apoptosis, or the association of proteasome, lysosome, and caspase pathways. A direct interaction was observed between RIG-I and 2B. The carboxyl-terminal amino acids 105 to 114 and amino acids 135 to 144 of 2B were essential for the reduction of RIG-I, while residues 105 to 114 were required for the interaction. These data suggest the antiviral role of RIG-I against FMDV and a novel antagonistic mechanism of FMDV that is mediated by 2B protein.
Importance: This study demonstrated that RIG-I could suppress FMDV replication during virus infection. FMDV infection increased the transcriptional expression of RIG-I, while it decreased RIG-I protein expression. FMDV 2B protein interacted with RIG-I and induced reduction of RIG-I. 2B-induced reduction of RIG-I was independent of the induction of the cleavage of eukaryotic translation initiation factor 4 gamma or cellular apoptosis. In addition, proteasome, lysosome, and caspase pathways were not involved in this process. This study provides new insight into the immune evasion mediated by FMDV and identifies 2B as an antagonistic factor for FMDV to evade the antiviral response.
Copyright © 2016 Zhu et al.
Figures





Similar articles
-
Foot-and-Mouth Disease Virus Antagonizes NOD2-Mediated Antiviral Effects by Inhibiting NOD2 Protein Expression.J Virol. 2019 May 15;93(11):e00124-19. doi: 10.1128/JVI.00124-19. Print 2019 Jun 1. J Virol. 2019. PMID: 30894473 Free PMC article.
-
Foot-and-mouth disease virus 3C protease cleaves NEMO to impair innate immune signaling.J Virol. 2012 Sep;86(17):9311-22. doi: 10.1128/JVI.00722-12. Epub 2012 Jun 20. J Virol. 2012. PMID: 22718831 Free PMC article.
-
Foot-and-mouth disease virus infection inhibits LGP2 protein expression to exaggerate inflammatory response and promote viral replication.Cell Death Dis. 2017 Apr 13;8(4):e2747. doi: 10.1038/cddis.2017.170. Cell Death Dis. 2017. PMID: 28406479 Free PMC article.
-
Evading the host immune response: how foot-and-mouth disease virus has become an effective pathogen.FEMS Immunol Med Microbiol. 2008 Jun;53(1):8-17. doi: 10.1111/j.1574-695X.2008.00409.x. Epub 2008 Apr 8. FEMS Immunol Med Microbiol. 2008. PMID: 18400012 Review.
-
Multifunctional roles of leader protein of foot-and-mouth disease viruses in suppressing host antiviral responses.Vet Res. 2015 Oct 28;46:127. doi: 10.1186/s13567-015-0273-1. Vet Res. 2015. PMID: 26511922 Free PMC article. Review.
Cited by
-
Basal Level p53 Suppresses Antiviral Immunity against Foot-and-Mouth Disease Virus.Viruses. 2019 Aug 7;11(8):727. doi: 10.3390/v11080727. Viruses. 2019. PMID: 31394868 Free PMC article.
-
African Swine Fever Virus Regulates Host Energy and Amino Acid Metabolism To Promote Viral Replication.J Virol. 2022 Feb 23;96(4):e0191921. doi: 10.1128/JVI.01919-21. Epub 2021 Dec 15. J Virol. 2022. PMID: 34908441 Free PMC article.
-
Impaired interferon response in senecavirus A infection and identification of 3Cpro as an antagonist.J Virol. 2024 Jul 23;98(7):e0058524. doi: 10.1128/jvi.00585-24. Epub 2024 Jun 13. J Virol. 2024. PMID: 38869319 Free PMC article.
-
Insights into Polyprotein Processing and RNA-Protein Interactions in Foot-and-Mouth Disease Virus Genome Replication.J Virol. 2023 May 31;97(5):e0017123. doi: 10.1128/jvi.00171-23. Epub 2023 May 8. J Virol. 2023. PMID: 37154761 Free PMC article.
-
Foot-and-mouth disease virus infection suppresses autophagy and NF-кB antiviral responses via degradation of ATG5-ATG12 by 3Cpro.Cell Death Dis. 2017 Jan 19;8(1):e2561. doi: 10.1038/cddis.2016.489. Cell Death Dis. 2017. PMID: 28102839 Free PMC article.
References
-
- Moffat K, Howell G, Knox C, Belsham GJ, Monaghan P, Ryan MD, Wileman T. 2005. Effects of foot-and-mouth disease virus nonstructural proteins on the structure and function of the early secretory pathway: 2BC but not 3A blocks endoplasmic reticulum-to-Golgi transport. J Virol 79:4382–4395. doi:10.1128/JVI.79.7.4382-4395.2005. - DOI - PMC - PubMed
-
- Moffat K, Knox C, Howell G, Clark SJ, Yang H, Belsham GJ, Ryan M, Wileman T. 2007. Inhibition of the secretory pathway by foot-and-mouth disease virus 2BC protein is reproduced by coexpression of 2B with 2C, and the site of inhibition is determined by the subcellular location of 2C. J Virol 81:1129–1139. doi:10.1128/JVI.00393-06. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

