Mucins and associated O-glycans based immunoprofile for stratification of colorectal polyps: clinical implication for improved colon surveillance
- PMID: 27705923
- PMCID: PMC5351688
- DOI: 10.18632/oncotarget.12347
Mucins and associated O-glycans based immunoprofile for stratification of colorectal polyps: clinical implication for improved colon surveillance
Abstract
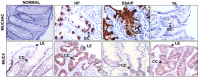
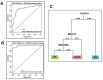
Sessile serrated adenoma/polyps (SSA/P) are premalignant lesions of colorectal cancer that are difficult to distinguish histologically from hyperplastic polyps (HP) of minimal to no malignant potential. Specific markers for differentiating SSA/P from HP can aid clinicians for optimizing colon surveillance intervals. The present study investigates the potential of mucins and associated O-glycans to distinguish SSA/P from HP. Expression of colonic mucins (MUC1, MUC4, MUC17, MUC2, and MUC5AC) and O-glycans [Sialyl LewisA (CA19-9) and Tn/Sialyl-Tn on MUC1] were analyzed in HP (n=33), SSA/P (n=39), and tubular adenoma (TA) (n=36) samples by immunohistochemistry. A significantly reduced expression of MUC4 (p=0.0066), elevated expression of MUC17 (p=0.0002), and MUC5AC (p<0.0001) was observed in SSA/P cases in comparison to HP cases. Interestingly, significantly higher number of SSA/P cases (p<0.0001) exhibited MUC5AC expression in the goblet cells as well as filled the crypt lumen compared to only goblet cells in majority of the HP cases. Improved diagnostic potential was revealed by multivariate logistic regression analysis where combinatorial panel of MUC5AC/MUC17 discriminated SSA/P from HP (SN/SP=85/82%). Finally, the decision tree model based marker panel (CA19-9/MUC17/MUC5AC) predicted HP, SSA/P and TA with SN/SP of 58%/95%, 79%/90% and 97%/83%, respectively. Overall, the mucin and associated O-glycan based panel defined in the present study could aid in discriminating SSA/P from HP to devise better colon surveillance strategies.
Keywords: O-glycans; hyperplastic polyps; mucins; sessile serrated adenoma/polyps; tubular adenomas.
Conflict of interest statement
Authors declare no conflict of interest.
Figures

Similar articles
-
MUC expression in hyperplastic and serrated colonic polyps: lack of specificity of MUC6.Am J Surg Pathol. 2011 May;35(5):742-9. doi: 10.1097/PAS.0b013e31821537a2. Am J Surg Pathol. 2011. PMID: 21490447
-
Mucins and associated glycan signatures in colon adenoma-carcinoma sequence: Prospective pathological implication(s) for early diagnosis of colon cancer.Cancer Lett. 2016 May 1;374(2):304-14. doi: 10.1016/j.canlet.2016.02.016. Epub 2016 Feb 16. Cancer Lett. 2016. PMID: 26898938 Free PMC article.
-
Immunohistochemical staining patterns of MUC1, MUC2, MUC4, and MUC5AC mucins in hyperplastic polyps, serrated adenomas, and traditional adenomas of the colorectum.J Histochem Cytochem. 1999 Aug;47(8):1039-48. doi: 10.1177/002215549904700808. J Histochem Cytochem. 1999. PMID: 10424888
-
Mucins and mucin binding proteins in colorectal cancer.Cancer Metastasis Rev. 2004 Jan-Jun;23(1-2):77-99. doi: 10.1023/a:1025815113599. Cancer Metastasis Rev. 2004. PMID: 15000151 Review.
-
Sessile serrated adenoma/polyps: Where are we at in 2016?World J Gastroenterol. 2016 Sep 14;22(34):7754-9. doi: 10.3748/wjg.v22.i34.7754. World J Gastroenterol. 2016. PMID: 27678358 Free PMC article. Review.
Cited by
-
Biomarkers for Early Detection of Colorectal Cancer: The Early Detection Research Network, a Framework for Clinical Translation.Cancer Epidemiol Biomarkers Prev. 2020 Dec;29(12):2431-2440. doi: 10.1158/1055-9965.EPI-20-0234. Epub 2020 Apr 16. Cancer Epidemiol Biomarkers Prev. 2020. PMID: 32299850 Free PMC article.
-
Expression of cyclooxygenase-2 and mucin 1 in colorectal cancer.Mol Clin Oncol. 2020 Nov;13(5):52. doi: 10.3892/mco.2020.2122. Epub 2020 Aug 20. Mol Clin Oncol. 2020. PMID: 32874582 Free PMC article.
-
The Mucin Family of Proteins: Candidates as Potential Biomarkers for Colon Cancer.Cancers (Basel). 2023 Feb 27;15(5):1491. doi: 10.3390/cancers15051491. Cancers (Basel). 2023. PMID: 36900282 Free PMC article. Review.
-
Mechanistic and Functional Shades of Mucins and Associated Glycans in Colon Cancer.Cancers (Basel). 2020 Mar 11;12(3):649. doi: 10.3390/cancers12030649. Cancers (Basel). 2020. PMID: 32168759 Free PMC article. Review.
-
Mucins as Potential Biomarkers for Early Detection of Cancer.Cancers (Basel). 2023 Mar 7;15(6):1640. doi: 10.3390/cancers15061640. Cancers (Basel). 2023. PMID: 36980526 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

