Risk of prenatal depression and stress treatment: alteration on serotonin system of offspring through exposure to Fluoxetine
- PMID: 27703173
- PMCID: PMC5050550
- DOI: 10.1038/srep33822
Risk of prenatal depression and stress treatment: alteration on serotonin system of offspring through exposure to Fluoxetine
Erratum in
-
Erratum: Risk of prenatal depression and stress treatment: alteration on serotonin system of offspring through exposure to Fluoxetine.Sci Rep. 2017 Feb 13;7:41344. doi: 10.1038/srep41344. Sci Rep. 2017. PMID: 28198797 Free PMC article. No abstract available.
Abstract
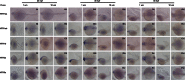
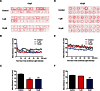
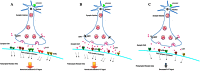
Fluoxetine is widely used to treat depression, including depression in pregnant and postpartum women. Studies suggest that fluoxetine may have adverse effects on offspring, presumably through its action on various serotonin receptors (HTRs). However, definitive evidence and the underlying mechanisms are largely unavailable. As initial steps towards establishing a human cellular and animal model, we analyzed the expression patterns of several HTRs through the differentiation of human induced pluripotent stem (hiPS) cells into neuronal cells, and analyzed expression pattern in zebrafish embryos. Treatment of zebrafish embryos with fluoxetine significantly blocked the expression of multiple HTRs. Furthermore, fluoxetine gave rise to a change in neuropsychology. Embryos treated with fluoxetine continued to exhibit abnormal behavior upto 12 days post fertilization due to changes in HTRs. These findings support a possible long-term risk of serotonin pathway alteration, possibly resulting from the "placental drug transfer".
Conflict of interest statement
The authors declare no competing financial interests.
Figures
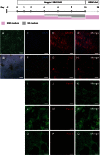
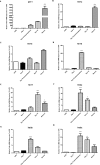
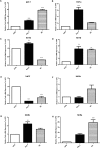
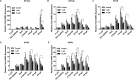

Similar articles
-
Tryptophan alleviates neuroendocrine and behavioral responses to stress in zebrafish.Behav Brain Res. 2020 Jan 27;378:112264. doi: 10.1016/j.bbr.2019.112264. Epub 2019 Sep 27. Behav Brain Res. 2020. PMID: 31568833
-
Fluoxetine coupled with zinc in a chronic mild stress model of depression: Providing a reservoir for optimum zinc signaling and neuronal remodeling.Pharmacol Biochem Behav. 2017 Sep;160:30-38. doi: 10.1016/j.pbb.2017.08.003. Epub 2017 Aug 9. Pharmacol Biochem Behav. 2017. PMID: 28801265
-
Developmental fluoxetine exposure increases behavioral despair and alters epigenetic regulation of the hippocampal BDNF gene in adult female offspring.Horm Behav. 2016 Apr;80:47-57. doi: 10.1016/j.yhbeh.2016.01.017. Epub 2016 Feb 1. Horm Behav. 2016. PMID: 26844865
-
Sex differences in animal models of depression and antidepressant response.Basic Clin Pharmacol Toxicol. 2010 Mar;106(3):226-33. doi: 10.1111/j.1742-7843.2009.00516.x. Epub 2009 Dec 30. Basic Clin Pharmacol Toxicol. 2010. PMID: 20050844 Review.
-
Anti-inflammatory, antiapoptotic, and antioxidant activity of fluoxetine.Pharmacol Res Perspect. 2016 Apr 7;4(3):e00231. doi: 10.1002/prp2.231. eCollection 2016 Jun. Pharmacol Res Perspect. 2016. PMID: 27433341 Free PMC article. Review.
Cited by
-
Pre-reproductive stress and fluoxetine treatment in rats affect offspring A-to-I RNA editing, gene expression and social behavior.Environ Epigenet. 2018 Aug 8;4(2):dvy021. doi: 10.1093/eep/dvy021. eCollection 2018 Apr. Environ Epigenet. 2018. PMID: 30109132 Free PMC article.
-
A zebrafish and mouse model for selective pruritus via direct activation of TRPA1.Elife. 2018 Mar 21;7:e32036. doi: 10.7554/eLife.32036. Elife. 2018. PMID: 29561265 Free PMC article.
-
Serotonin neuromodulation directs optic nerve regeneration.bioRxiv [Preprint]. 2024 Aug 13:2024.08.12.607648. doi: 10.1101/2024.08.12.607648. bioRxiv. 2024. PMID: 39185204 Free PMC article. Preprint.
-
Prenatal SSRI Exposure Increases the Risk of Autism in Rodents via Aggravated Oxidative Stress and Neurochemical Changes in the Brain.Metabolites. 2023 Feb 20;13(2):310. doi: 10.3390/metabo13020310. Metabolites. 2023. PMID: 36837929 Free PMC article.
-
THC-induced behavioral stereotypy in zebrafish as a model of psychosis-like behavior.Sci Rep. 2021 Aug 3;11(1):15693. doi: 10.1038/s41598-021-95016-4. Sci Rep. 2021. PMID: 34344922 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

