Enhanced sensitivity to drugs of abuse and palatable foods following maternal overnutrition
- PMID: 27701408
- PMCID: PMC5315546
- DOI: 10.1038/tp.2016.176
Enhanced sensitivity to drugs of abuse and palatable foods following maternal overnutrition
Abstract
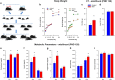
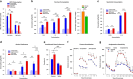
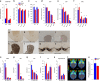
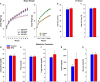
Epidemiological studies have shown an association between maternal overnutrition and increased risk of the progeny for the development of obesity as well as psychiatric disorders. Animal studies have shown results regarding maternal high-fat diet (HFD) and a greater risk of the offspring to develop obesity. However, it still remains unknown whether maternal HFD can program the central reward system in such a way that it will imprint long-term changes that will predispose the offspring to addictive-like behaviors that may lead to obesity. We exposed female dams to either laboratory chow or HFD for a period of 9 weeks: 3 weeks before conception, during gestation and lactation. Offspring born to either control or HFD-exposed dams were examined in behavioral, neurochemical, neuroanatomical, metabolic and positron emission tomography (PET) scan tests. Our results demonstrate that HFD offspring compared with controls consume more alcohol, exhibit increased sensitivity to amphetamine and show greater conditioned place preference to cocaine. In addition, maternal HFD leads to increased preference to sucrose as well as to HFD while leaving the general feeding behavior intact. The hedonic behavioral alterations are accompanied by reduction of striatal dopamine and by increased dopamine 2 receptors in the same brain region as evaluated by post-mortem neurochemical, immunohistochemical as well as PET analyses. Taken together, our data suggest that maternal overnutrition predisposes the offspring to develop hedonic-like behaviors to both drugs of abuse as well as palatable foods and that these types of behaviors may share common neuronal underlying mechanisms that can lead to obesity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Maternal Over- and Malnutrition and Increased Risk for Addictive and Eating Disorders in the Offspring.Nutrients. 2023 Feb 22;15(5):1095. doi: 10.3390/nu15051095. Nutrients. 2023. PMID: 36904093 Free PMC article. Review.
-
Maternal high-fat diet and obesity impact palatable food intake and dopamine signaling in nonhuman primate offspring.Obesity (Silver Spring). 2015 Nov;23(11):2157-64. doi: 10.1002/oby.21306. Obesity (Silver Spring). 2015. PMID: 26530932 Free PMC article.
-
Differential Effects of Maternal High Fat Diet During Pregnancy and Lactation on Taste Preferences in Rats.Nutrients. 2020 Nov 20;12(11):3553. doi: 10.3390/nu12113553. Nutrients. 2020. PMID: 33233529 Free PMC article.
-
Maternal nicotine exposure during lactation alters food preference, anxiety-like behavior and the brain dopaminergic reward system in the adult rat offspring.Physiol Behav. 2015 Oct 1;149:131-41. doi: 10.1016/j.physbeh.2015.05.040. Epub 2015 Jun 3. Physiol Behav. 2015. PMID: 26048299
-
Preclinical evidence for the addiction potential of highly palatable foods: Current developments related to maternal influence.Appetite. 2017 Aug 1;115:19-27. doi: 10.1016/j.appet.2016.12.019. Epub 2016 Dec 15. Appetite. 2017. PMID: 27989563 Review.
Cited by
-
Maternal Over- and Malnutrition and Increased Risk for Addictive and Eating Disorders in the Offspring.Nutrients. 2023 Feb 22;15(5):1095. doi: 10.3390/nu15051095. Nutrients. 2023. PMID: 36904093 Free PMC article. Review.
-
The Influence of Maternal Metabolic State and Nutrition on Offspring Neurobehavioral Development: A Focus on Preclinical Models.Biol Psychiatry Cogn Neurosci Neuroimaging. 2022 May;7(5):450-460. doi: 10.1016/j.bpsc.2021.11.014. Epub 2021 Dec 13. Biol Psychiatry Cogn Neurosci Neuroimaging. 2022. PMID: 34915175 Free PMC article. Review.
-
Adolescent microglia play a role in executive function in male mice exposed to perinatal high fat diet.Brain Behav Immun. 2020 Feb;84:80-89. doi: 10.1016/j.bbi.2019.11.010. Epub 2019 Nov 23. Brain Behav Immun. 2020. PMID: 31765789 Free PMC article.
-
Transgenerational Susceptibility to Food Addiction-Like Behavior in Rats Associates to a Decrease of the Anti-Inflammatory IL-10 in Plasma.Neurochem Res. 2022 Oct;47(10):3093-3103. doi: 10.1007/s11064-022-03660-7. Epub 2022 Jun 29. Neurochem Res. 2022. PMID: 35767136
-
Maternal cafeteria diet exposure primes depression-like behavior in the offspring evoking lower brain volume related to changes in synaptic terminals and gliosis.Transl Psychiatry. 2021 Jan 14;11(1):53. doi: 10.1038/s41398-020-01157-x. Transl Psychiatry. 2021. PMID: 33446642 Free PMC article.
References
-
- Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet 2012; 379: 55–70. - PubMed
-
- WHO2012. Obesity and Overweight. In: Fact sheet N°311 http://www.who.int/mediacentre/factsheets/fs311/en/index.html.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

