ALB3 Insertase Mediates Cytochrome b6 Co-translational Import into the Thylakoid Membrane
- PMID: 27698412
- PMCID: PMC5048292
- DOI: 10.1038/srep34557
ALB3 Insertase Mediates Cytochrome b6 Co-translational Import into the Thylakoid Membrane
Abstract
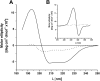
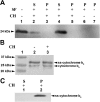
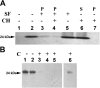
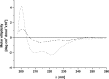
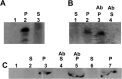
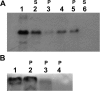
The cytochrome b6 f complex occupies an electrochemically central position in the electron-transport chain bridging the photosynthetic reaction center of PS I and PS II. In plants, the subunits of these thylakoid membrane protein complexes are both chloroplast and nuclear encoded. How the chloroplast-encoded subunits of multi-spanning cytochrome b6 are targeted and inserted into the thylakoid membrane is not fully understood. Experimental approaches to evaluate the cytochrome b6 import mechanism in vivo have been limited to bacterial membranes and were not a part of the chloroplast environment. To evaluate the mechanism governing cytochrome b6 integration in vivo, we performed a comparative analysis of both native and synthetic cytochrome b6 insertion into purified thylakoids. Using biophysical and biochemical methods, we show that cytochrome b6 insertion into the thylakoid membrane is a non-spontaneous co-translational process that involves ALB3 insertase. Furthermore, we provided evidence that CSP41 (chloroplast stem-loop-binding protein of 41 kDa) interacts with RNC-cytochrome b6 complexes, and may be involved in cytochrome b6 (petB) transcript stabilization or processing.
Conflict of interest statement
Małgorzata Piskozub has paid employment at Amplicon Sp. z o. o. The following authors have no competing interests: Rafał Bartoszewski, Bożena Króliczewska, Jarosław Króliczewski.
Figures


Similar articles
-
Chloroplast PetD protein: evidence for SRP/Alb3-dependent insertion into the thylakoid membrane.BMC Plant Biol. 2017 Nov 21;17(1):213. doi: 10.1186/s12870-017-1176-2. BMC Plant Biol. 2017. PMID: 29162052 Free PMC article.
-
Ribosome nascent chain complexes of the chloroplast-encoded cytochrome b6 thylakoid membrane protein interact with cpSRP54 but not with cpSecY.J Bioenerg Biomembr. 2015 Jun;47(3):265-78. doi: 10.1007/s10863-014-9598-0. Epub 2015 Jan 6. J Bioenerg Biomembr. 2015. PMID: 25561393 Free PMC article.
-
In vitro reconstitution of co-translational D1 insertion reveals a role of the cpSec-Alb3 translocase and Vipp1 in photosystem II biogenesis.Biochem J. 2015 Jun 1;468(2):315-24. doi: 10.1042/BJ20141425. Biochem J. 2015. PMID: 25803492
-
Mechanisms of protein import into thylakoids of chloroplasts.Biol Chem. 2007 Sep;388(9):907-15. doi: 10.1515/BC.2007.111. Biol Chem. 2007. PMID: 17696774 Review.
-
How to build functional thylakoid membranes: from plastid transcription to protein complex assembly.Planta. 2013 Feb;237(2):413-28. doi: 10.1007/s00425-012-1752-5. Epub 2012 Sep 14. Planta. 2013. PMID: 22976450 Free PMC article. Review.
Cited by
-
De-etiolation-induced protein 1 (DEIP1) mediates assembly of the cytochrome b6f complex in Arabidopsis.Nat Commun. 2022 Jul 13;13(1):4045. doi: 10.1038/s41467-022-31758-7. Nat Commun. 2022. PMID: 35831297 Free PMC article.
-
Transient local secondary structure in the intrinsically disordered C-term of the Albino3 insertase.Biophys J. 2021 Nov 16;120(22):4992-5004. doi: 10.1016/j.bpj.2021.10.013. Epub 2021 Oct 16. Biophys J. 2021. PMID: 34662559 Free PMC article.
-
Structural disorder in plant proteins: where plasticity meets sessility.Cell Mol Life Sci. 2017 Sep;74(17):3119-3147. doi: 10.1007/s00018-017-2557-2. Epub 2017 Jun 22. Cell Mol Life Sci. 2017. PMID: 28643166 Free PMC article. Review.
-
OsALB3 Is Required for Chloroplast Development by Promoting the Accumulation of Light-Harvesting Chlorophyll-Binding Proteins in Rice.Plants (Basel). 2023 Nov 28;12(23):4003. doi: 10.3390/plants12234003. Plants (Basel). 2023. PMID: 38068638 Free PMC article.
-
Chloroplast Translation: Structural and Functional Organization, Operational Control, and Regulation.Plant Cell. 2018 Apr;30(4):745-770. doi: 10.1105/tpc.18.00016. Epub 2018 Apr 2. Plant Cell. 2018. PMID: 29610211 Free PMC article. Review.
References
-
- Cramer W. A., Yamashita E. & Hasan S. S. In Encyclopedia of Biological Chemistry Vol. 4 (eds Lane M. D. & Lennarz W. J.) 167–171 (Academic Press, Waltham MA, 2013).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

