TRIMs and Galectins Globally Cooperate and TRIM16 and Galectin-3 Co-direct Autophagy in Endomembrane Damage Homeostasis
- PMID: 27693506
- PMCID: PMC5104201
- DOI: 10.1016/j.devcel.2016.08.003
TRIMs and Galectins Globally Cooperate and TRIM16 and Galectin-3 Co-direct Autophagy in Endomembrane Damage Homeostasis
Abstract
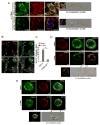
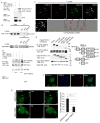
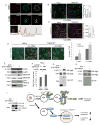
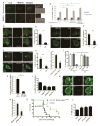
Selective autophagy performs an array of tasks to maintain intracellular homeostasis, sterility, and organellar and cellular functionality. The fidelity of these processes depends on precise target recognition and limited activation of the autophagy apparatus in a localized fashion. Here we describe cooperation in such processes between the TRIM family and Galectin family of proteins. TRIMs, which are E3 ubiquitin ligases, displayed propensity to associate with Galectins. One specific TRIM, TRIM16, interacted with Galectin-3 in a ULK1-dependent manner. TRIM16, through integration of Galectin- and ubiquitin-based processes, coordinated recognition of membrane damage with mobilization of the core autophagy regulators ATG16L1, ULK1, and Beclin 1 in response to damaged endomembranes. TRIM16 affected mTOR, interacted with TFEB, and influenced TFEB's nuclear translocation. The cooperation between TRIM16 and Galectin-3 in targeting and activation of selective autophagy protects cells from lysosomal damage and Mycobacterium tuberculosis invasion.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures



Comment in
-
A TRIM16-Galactin3 Complex Mediates Autophagy of Damaged Endomembranes.Dev Cell. 2016 Oct 10;39(1):1-2. doi: 10.1016/j.devcel.2016.09.025. Dev Cell. 2016. PMID: 27728777
Similar articles
-
Galectins and TRIMs directly interact and orchestrate autophagic response to endomembrane damage.Autophagy. 2017 Jun 3;13(6):1086-1087. doi: 10.1080/15548627.2017.1307487. Epub 2017 Apr 3. Autophagy. 2017. PMID: 28368693 Free PMC article.
-
TRIM16 controls assembly and degradation of protein aggregates by modulating the p62-NRF2 axis and autophagy.EMBO J. 2018 Sep 14;37(18):e98358. doi: 10.15252/embj.201798358. Epub 2018 Aug 24. EMBO J. 2018. PMID: 30143514 Free PMC article.
-
PRKAA2, MTOR, and TFEB in the regulation of lysosomal damage response and autophagy.J Mol Med (Berl). 2024 Mar;102(3):287-311. doi: 10.1007/s00109-023-02411-7. Epub 2024 Jan 6. J Mol Med (Berl). 2024. PMID: 38183492 Review.
-
SMURF1 controls the PPP3/calcineurin complex and TFEB at a regulatory node for lysosomal biogenesis.Autophagy. 2024 Apr;20(4):735-751. doi: 10.1080/15548627.2023.2267413. Epub 2023 Nov 1. Autophagy. 2024. PMID: 37909662 Free PMC article.
-
The Roles of Ubiquitin in Mediating Autophagy.Cells. 2020 Sep 2;9(9):2025. doi: 10.3390/cells9092025. Cells. 2020. PMID: 32887506 Free PMC article. Review.
Cited by
-
Fbxo2 mediates clearance of damaged lysosomes and modifies neurodegeneration in the Niemann-Pick C brain.JCI Insight. 2020 Oct 15;5(20):e136676. doi: 10.1172/jci.insight.136676. JCI Insight. 2020. PMID: 32931479 Free PMC article.
-
The role of ubiquitination and deubiquitination in cancer metabolism.Mol Cancer. 2020 Oct 1;19(1):146. doi: 10.1186/s12943-020-01262-x. Mol Cancer. 2020. PMID: 33004065 Free PMC article. Review.
-
Galectin-3, Possible Role in Pathogenesis of Periodontal Diseases and Potential Therapeutic Target.Front Pharmacol. 2021 Mar 19;12:638258. doi: 10.3389/fphar.2021.638258. eCollection 2021. Front Pharmacol. 2021. PMID: 33815121 Free PMC article. Review.
-
Galectin-3 Coordinates a Cellular System for Lysosomal Repair and Removal.Dev Cell. 2020 Jan 6;52(1):69-87.e8. doi: 10.1016/j.devcel.2019.10.025. Epub 2019 Dec 5. Dev Cell. 2020. PMID: 31813797 Free PMC article.
-
Biological Functions of Autophagy Genes: A Disease Perspective.Cell. 2019 Jan 10;176(1-2):11-42. doi: 10.1016/j.cell.2018.09.048. Cell. 2019. PMID: 30633901 Free PMC article. Review.
References
-
- Antonioli M, Albiero F, Nazio F, Vescovo T, Perdomo AB, Corazzari M, Marsella C, Piselli P, Gretzmeier C, Dengjel J, et al. AMBRA1 interplay with cullin E3 ubiquitin ligases regulates autophagy dynamics. Dev Cell. 2014;31:734–746. - PubMed
-
- Beatty WL, Rhoades ER, Hsu DK, Liu FT, Russell DG. Association of a macrophage galactoside-binding protein with Mycobacterium-containing phagosomes. Cell Microbiol. 2002;4:167–176. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

