Engineering T Cells with Customized Therapeutic Response Programs Using Synthetic Notch Receptors
- PMID: 27693353
- PMCID: PMC5072533
- DOI: 10.1016/j.cell.2016.09.011
Engineering T Cells with Customized Therapeutic Response Programs Using Synthetic Notch Receptors
Abstract
Redirecting T cells to attack cancer using engineered chimeric receptors provides powerful new therapeutic capabilities. However, the effectiveness of therapeutic T cells is constrained by the endogenous T cell response: certain facets of natural response programs can be toxic, whereas other responses, such as the ability to overcome tumor immunosuppression, are absent. Thus, the efficacy and safety of therapeutic cells could be improved if we could custom sculpt immune cell responses. Synthetic Notch (synNotch) receptors induce transcriptional activation in response to recognition of user-specified antigens. We show that synNotch receptors can be used to sculpt custom response programs in primary T cells: they can drive a la carte cytokine secretion profiles, biased T cell differentiation, and local delivery of non-native therapeutic payloads, such as antibodies, in response to antigen. SynNotch T cells can thus be used as a general platform to recognize and remodel local microenvironments associated with diverse diseases.
Keywords: CARs; T cells; cancer; cellular engineering; immunotherapy; synthetic Notch; synthetic biology.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
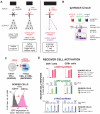

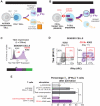
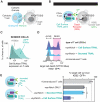
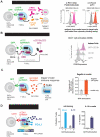
Comment in
-
Customizing Functionality and Payload Delivery for Receptor-Engineered T Cells.Cell. 2016 Oct 6;167(2):304-306. doi: 10.1016/j.cell.2016.09.033. Cell. 2016. PMID: 27716501 Free PMC article.
Similar articles
-
Engineering Customized Cell Sensing and Response Behaviors Using Synthetic Notch Receptors.Cell. 2016 Feb 11;164(4):780-91. doi: 10.1016/j.cell.2016.01.012. Epub 2016 Jan 28. Cell. 2016. PMID: 26830878 Free PMC article.
-
Preclinical targeting of human T-cell malignancies using CD4-specific chimeric antigen receptor (CAR)-engineered T cells.Leukemia. 2016 Mar;30(3):701-7. doi: 10.1038/leu.2015.311. Epub 2015 Nov 3. Leukemia. 2016. PMID: 26526988
-
Engineering of an enhanced synthetic Notch receptor by reducing ligand-independent activation.Commun Biol. 2020 Mar 13;3(1):116. doi: 10.1038/s42003-020-0848-x. Commun Biol. 2020. PMID: 32170210 Free PMC article.
-
Synthetic Immunology: Hacking Immune Cells to Expand Their Therapeutic Capabilities.Annu Rev Immunol. 2017 Apr 26;35:229-253. doi: 10.1146/annurev-immunol-051116-052302. Annu Rev Immunol. 2017. PMID: 28446063 Free PMC article. Review.
-
Programmable synthetic receptors: the next-generation of cell and gene therapies.Signal Transduct Target Ther. 2024 Jan 3;9(1):7. doi: 10.1038/s41392-023-01680-5. Signal Transduct Target Ther. 2024. PMID: 38167329 Free PMC article. Review.
Cited by
-
Navigating CAR-T cells through the solid-tumour microenvironment.Nat Rev Drug Discov. 2021 Jul;20(7):531-550. doi: 10.1038/s41573-021-00189-2. Epub 2021 May 10. Nat Rev Drug Discov. 2021. PMID: 33972771 Review.
-
Programmable protein circuit design.Cell. 2021 Apr 29;184(9):2284-2301. doi: 10.1016/j.cell.2021.03.007. Epub 2021 Apr 12. Cell. 2021. PMID: 33848464 Free PMC article. Review.
-
Synthetic neuromorphic computing in living cells.Nat Commun. 2022 Sep 24;13(1):5602. doi: 10.1038/s41467-022-33288-8. Nat Commun. 2022. PMID: 36153343 Free PMC article.
-
CAR-T cells and BiTEs in solid tumors: challenges and perspectives.J Hematol Oncol. 2021 Apr 19;14(1):65. doi: 10.1186/s13045-021-01067-5. J Hematol Oncol. 2021. PMID: 33874996 Free PMC article. Review.
-
Discovery and validation of human genomic safe harbor sites for gene and cell therapies.Cell Rep Methods. 2022 Jan 14;2(1):100154. doi: 10.1016/j.crmeth.2021.100154. eCollection 2022 Jan 24. Cell Rep Methods. 2022. PMID: 35474867 Free PMC article.
References
-
- Akira S, Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4:499–511. - PubMed
-
- Anderson R, Macdonald I, Corbett T, Hacking G, Lowdell MW, Prentice HG. Construction and biological characterization of an interleukin-12 fusion protein (Flexi-12): delivery to acute myeloid leukemic blasts using adeno-associated virus. Hum. Gene Ther. 1997;8:1125–1135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

