Biomarkers of Environmental Enteropathy, Inflammation, Stunting, and Impaired Growth in Children in Northeast Brazil
- PMID: 27690129
- PMCID: PMC5045163
- DOI: 10.1371/journal.pone.0158772
Biomarkers of Environmental Enteropathy, Inflammation, Stunting, and Impaired Growth in Children in Northeast Brazil
Abstract
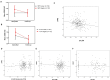
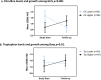
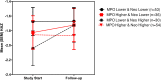
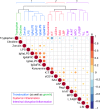
Critical to the design and assessment of interventions for enteropathy and its developmental consequences in children living in impoverished conditions are non-invasive biomarkers that can detect intestinal damage and predict its effects on growth and development. We therefore assessed fecal, urinary and systemic biomarkers of enteropathy and growth predictors in 375 6-26 month-old children with varying degrees of malnutrition (stunting or wasting) in Northeast Brazil. 301 of these children returned for followup anthropometry after 2-6m. Biomarkers that correlated with stunting included plasma IgA anti-LPS and anti-FliC, zonulin (if >12m old), and intestinal FABP (I-FABP, suggesting prior barrier disruption); and with citrulline, tryptophan and with lower serum amyloid A (SAA) (suggesting impaired defenses). In contrast, subsequent growth was predicted in those with higher fecal MPO or A1AT and also by higher L/M, plasma LPS, I-FABP and SAA (showing intestinal barrier disruption and inflammation). Better growth was predicted in girls with higher plasma citrulline and in boys with higher plasma tryptophan. Interactions were also seen with fecal MPO and neopterin in predicting subsequent growth impairment. Biomarkers clustered into markers of 1) functional intestinal barrier disruption and translocation, 2) structural intestinal barrier disruption and inflammation and 3) systemic inflammation. Principle components pathway analyses also showed that L/M with %L, I-FABP and MPO associate with impaired growth, while also (like MPO) associating with a systemic inflammation cluster of kynurenine, LBP, sCD14, SAA and K/T. Systemic evidence of LPS translocation associated with stunting, while markers of barrier disruption or repair (A1AT and Reg1 with low zonulin) associated with fecal MPO and neopterin. We conclude that key noninvasive biomarkers of intestinal barrier disruption, LPS translocation and of intestinal and systemic inflammation can help elucidate how we recognize, understand, and assess effective interventions for enteropathy and its growth and developmental consequences in children in impoverished settings.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Causal Pathways from Enteropathogens to Environmental Enteropathy: Findings from the MAL-ED Birth Cohort Study.EBioMedicine. 2017 Apr;18:109-117. doi: 10.1016/j.ebiom.2017.02.024. Epub 2017 Mar 8. EBioMedicine. 2017. PMID: 28396264 Free PMC article.
-
Environmental enteric dysfunction and systemic inflammation predict reduced weight but not length gain in rural Bangladeshi children.Br J Nutr. 2018 Feb;119(4):407-414. doi: 10.1017/S0007114517003683. Br J Nutr. 2018. PMID: 29498344 Clinical Trial.
-
Mycotoxins were not associated with environmental enteropathy in a cohort of Tanzanian children.Risk Anal. 2023 Apr;43(4):860-866. doi: 10.1111/risa.13956. Epub 2022 May 26. Risk Anal. 2023. PMID: 35618664
-
Relevance of biomarkers indicating gut damage and microbial translocation in people living with HIV.Front Immunol. 2023 Apr 21;14:1173956. doi: 10.3389/fimmu.2023.1173956. eCollection 2023. Front Immunol. 2023. PMID: 37153621 Free PMC article. Review.
-
Early-life enteric infections: relation between chronic systemic inflammation and poor cognition in children.Nutr Rev. 2016 Jun;74(6):374-86. doi: 10.1093/nutrit/nuw008. Epub 2016 May 3. Nutr Rev. 2016. PMID: 27142301 Free PMC article. Review.
Cited by
-
Environmental enteric dysfunction: a review of potential mechanisms, consequences and management strategies.BMC Med. 2019 Nov 25;17(1):181. doi: 10.1186/s12916-019-1417-3. BMC Med. 2019. PMID: 31760941 Free PMC article. Review.
-
Assessment of Machine Learning Detection of Environmental Enteropathy and Celiac Disease in Children.JAMA Netw Open. 2019 Jun 5;2(6):e195822. doi: 10.1001/jamanetworkopen.2019.5822. JAMA Netw Open. 2019. PMID: 31199451 Free PMC article.
-
Critical Role of Zinc in a New Murine Model of Enterotoxigenic Escherichia coli Diarrhea.Infect Immun. 2018 Jun 21;86(7):e00183-18. doi: 10.1128/IAI.00183-18. Print 2018 Jul. Infect Immun. 2018. PMID: 29661930 Free PMC article.
-
Paediatric schistosomiasis: What we know and what we need to know.PLoS Negl Trop Dis. 2018 Feb 8;12(2):e0006144. doi: 10.1371/journal.pntd.0006144. eCollection 2018 Feb. PLoS Negl Trop Dis. 2018. PMID: 29420537 Free PMC article. Review.
-
Effects of improved water, sanitation, and hygiene and improved complementary feeding on environmental enteric dysfunction in children in rural Zimbabwe: A cluster-randomized controlled trial.PLoS Negl Trop Dis. 2020 Feb 14;14(2):e0007963. doi: 10.1371/journal.pntd.0007963. eCollection 2020 Feb. PLoS Negl Trop Dis. 2020. PMID: 32059011 Free PMC article. Clinical Trial.
References
-
- Mendez MA, Adair LS. Severity and timing of stunting in the first two years of life affect performance on cognitive tests in late childhood. J Nutr. 1999;129(8):1555–62. Epub 1999/07/27. . - PubMed
-
- Stein AD, Wang M, DiGirolamo A, Grajeda R, Ramakrishnan U, Ramirez-Zea M, et al. Nutritional supplementation in early childhood, schooling, and intellectual functioning in adulthood: a prospective study in Guatemala. Archives of pediatrics & adolescent medicine. 2008;162(7):612–8. Epub 2008/07/09. 10.1001/archpedi.162.7.612 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

