Elastography in Chronic Liver Disease: Modalities, Techniques, Limitations, and Future Directions
- PMID: 27689833
- PMCID: PMC5584553
- DOI: 10.1148/rg.2016160042
Elastography in Chronic Liver Disease: Modalities, Techniques, Limitations, and Future Directions
Abstract
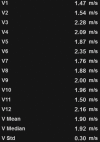
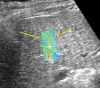
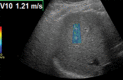
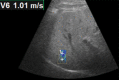
Chronic liver disease has multiple causes, many of which are increasing in prevalence. The final common pathway of chronic liver disease is tissue destruction and attempted regeneration, a pathway that triggers fibrosis and eventual cirrhosis. Assessment of fibrosis is important not only for diagnosis but also for management, prognostic evaluation, and follow-up of patients with chronic liver disease. Although liver biopsy has traditionally been considered the reference standard for assessment of liver fibrosis, noninvasive techniques are the emerging focus in this field. Ultrasound-based elastography and magnetic resonance (MR) elastography are gaining popularity as the modalities of choice for quantifying hepatic fibrosis. These techniques have been proven superior to conventional cross-sectional imaging for evaluation of fibrosis, especially in the precirrhotic stages. Moreover, elastography has added utility in the follow-up of previously diagnosed fibrosis, the assessment of treatment response, evaluation for the presence of portal hypertension (spleen elastography), and evaluation of patients with unexplained portal hypertension. In this article, a brief overview is provided of chronic liver disease and the tools used for its diagnosis. Ultrasound-based elastography and MR elastography are explored in depth, including a brief glimpse into the evolution of elastography. Elastography is based on the principle of measuring tissue response to a known mechanical stimulus. Specific elastographic techniques used to exploit this principle include MR elastography and ultrasonography-based static or quasistatic strain imaging, one-dimensional transient elastography, point shear-wave elastography, and supersonic shear-wave elastography. The advantages, limitations, and pitfalls of each modality are emphasized. ©RSNA, 2016.
Figures

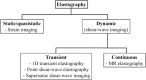
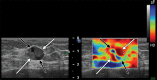
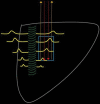
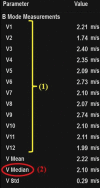
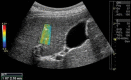




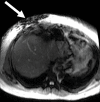
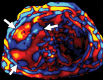
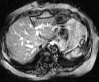
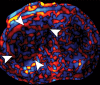
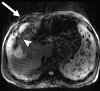
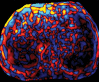
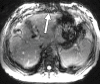
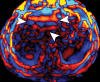
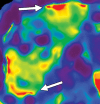
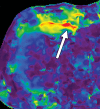
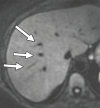
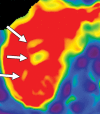
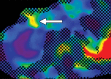
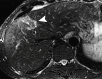
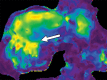
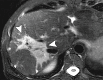
Comment in
-
Invited Commentary on "Elastography in Chronic Liver Disease".Radiographics. 2016 Nov-Dec;36(7):2007-2009. doi: 10.1148/rg.2016160182. Epub 2016 Sep 26. Radiographics. 2016. PMID: 27689832 No abstract available.
Similar articles
-
Ultrasound Shear Wave Elastography for Liver Disease. A Critical Appraisal of the Many Actors on the Stage.Ultraschall Med. 2016 Feb;37(1):1-5. doi: 10.1055/s-0035-1567037. Epub 2016 Feb 12. Ultraschall Med. 2016. PMID: 26871407
-
The role of ultrasound elastographic techniques in chronic liver disease: current status and future perspectives.Eur J Radiol. 2014 Mar;83(3):450-5. doi: 10.1016/j.ejrad.2013.06.009. Epub 2013 Jul 24. Eur J Radiol. 2014. PMID: 23891139 Review.
-
Quantitative Elastography Methods in Liver Disease: Current Evidence and Future Directions.Radiology. 2018 Mar;286(3):738-763. doi: 10.1148/radiol.2018170601. Radiology. 2018. PMID: 29461949 Free PMC article. Review.
-
Ultrasound elastography in liver.Diagn Interv Imaging. 2013 May;94(5):515-34. doi: 10.1016/j.diii.2013.02.005. Epub 2013 Apr 24. Diagn Interv Imaging. 2013. PMID: 23623211 Review.
-
Liver elastography - an update.Med Ultrason. 2013 Dec;15(4):304-14. doi: 10.11152/mu.2013.2066.154.isp23. Med Ultrason. 2013. PMID: 24286095 Review.
Cited by
-
Liver Function-How to Screen and to Diagnose: Insights from Personal Experiences, Controlled Clinical Studies and Future Perspectives.J Pers Med. 2022 Oct 5;12(10):1657. doi: 10.3390/jpm12101657. J Pers Med. 2022. PMID: 36294796 Free PMC article. Review.
-
Measurement of splenic stiffness by 2D-shear wave elastography in patients with extrahepatic portal vein obstruction.Br J Radiol. 2018 Dec;91(1092):20180401. doi: 10.1259/bjr.20180401. Epub 2018 Sep 18. Br J Radiol. 2018. PMID: 30226081 Free PMC article.
-
Diagnostic Performance of Greyscale Ultrasound in Detecting Fatty Liver Disease in a Type 2 Diabetes Population Using FibroScan as the Reference Standard.Cureus. 2023 Jun 21;15(6):e40756. doi: 10.7759/cureus.40756. eCollection 2023 Jun. Cureus. 2023. PMID: 37350981 Free PMC article.
-
Evaluation of the plantar fascia in patients with diabetes mellitus: the role of sonoelastography.Pol J Radiol. 2022 Sep 9;87:e500-e505. doi: 10.5114/pjr.2022.119474. eCollection 2022. Pol J Radiol. 2022. PMID: 36250143 Free PMC article.
-
Assessing significant fibrosis using imaging-based elastography in chronic hepatitis B patients: Pilot study.World J Gastroenterol. 2019 Jul 7;25(25):3256-3267. doi: 10.3748/wjg.v25.i25.3256. World J Gastroenterol. 2019. PMID: 31333316 Free PMC article.
References
-
- Sebastiani G, Castera L, Halfon P, et al. . The impact of liver disease aetiology and the stages of hepatic fibrosis on the performance of non-invasive fibrosis biomarkers: an international study of 2411 cases. Aliment Pharmacol Ther 2011;34(10):1202–1216. - PubMed
-
- Centers for Disease Control and Prevention . Viral hepatitis. Centers for Disease Control and Prevention website. http://www.cdc.gov/hepatitis/index.htm. Updated September 18, 2014. Accessed March 1, 2016.
-
- Friedman SL. Liver fibrosis: from bench to bedside. J Hepatol 2003;38(suppl 1):S38–S53. - PubMed
-
- Nagaoki Y, Aikata H, Nakano N, et al. . Development of hepatocellular carcinoma in patients with hepatitis C virus infection who achieved sustained virological response following interferon therapy: a large-scale, long-term cohort study. J Gastroenterol Hepatol 2016;31(5):1009–1015. - PubMed
-
- Ko CJ, Lin PY, Lin KH, Lin CC, Chen YL. Presence of fibrosis is predictive of postoperative survival in patients with small hepatocellular carcinoma. Hepatogastroenterology 2014;61(136):2295–2300. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

