Autophagy Activation by Transcription Factor EB (TFEB) in Striatum of HDQ175/Q7 Mice
- PMID: 27689619
- PMCID: PMC5088406
- DOI: 10.3233/JHD-160211
Autophagy Activation by Transcription Factor EB (TFEB) in Striatum of HDQ175/Q7 Mice
Abstract
Background: Mutant huntingtin (mHTT) is encoded by the Huntington's disease (HD) gene and its accumulation in the brain contributes to HD pathogenesis. Reducing mHTT levels through activation of the autophagosome-lysosomal pathway may have therapeutic benefit. Transcription factor EB (TFEB) regulates lysosome biogenesis and autophagy.
Objective: To examine if increasing TFEB protein levels in HD mouse striatum induces autophagy and influences mHTT levels.
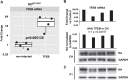
Methods: We introduced cDNA encoding TFEB with an HA tag (TFEB-HA) under the control of neuron specific synapsin 1 promoter into the striatum of 3 month old HDQ175/Q7 mice using adeno-associated virus AAV2/9. The levels of exogenous TFEB were analyzed using qPCR and Western blot. Proteins involved in autophagy, levels of huntingtin, and striatal-enriched proteins were examined using biochemical and/or immunohistochemical methods.
Results: In HD mice expressing TFEB-HA, HA immunoreactivity distributed throughout the striatum in neuronal cell bodies and processes and preferentially in neuronal nuclei and overlapped with a loss of DARPP32 immunoreactivity. TFEB-HA mRNA and protein were detected in striatal lysates. There were increased levels of proteins involved with autophagosome/lysosome activity including LAMP-2A, LC3II, and cathepsin D and reduced levels of mutant HTT and the striatal enriched proteins DARPP32 and PDE10A. Compared to WT mice, HDQ175/Q7 mice had elevated levels of the ER stress protein GRP78/BiP and with TFEB-HA expression, increased levels of the astrocyte marker GFAP and pro-caspase 3.
Conclusion: These results suggest that TFEB expression in the striatum of HDQ175/Q7 mice stimulates autophagy and lysosome activity, and lowers mHTT, but may also increase a neuronal stress response.
Keywords: Adeno associated virus; GRP78/BiP; Huntington’s disease; LC3; TFEB; autophagy; huntingtin; neurodegeneration; striatum.
Figures




Similar articles
-
Effects of Exogenous NUB1 Expression in the Striatum of HDQ175/Q7 Mice.J Huntingtons Dis. 2016 Jun 13;5(2):163-74. doi: 10.3233/JHD-160195. J Huntingtons Dis. 2016. PMID: 27314618
-
Pramipexole reduces soluble mutant huntingtin and protects striatal neurons through dopamine D3 receptors in a genetic model of Huntington's disease.Exp Neurol. 2018 Jan;299(Pt A):137-147. doi: 10.1016/j.expneurol.2017.10.019. Epub 2017 Oct 19. Exp Neurol. 2018. PMID: 29056363
-
Targeting the UPR transcription factor XBP1 protects against Huntington's disease through the regulation of FoxO1 and autophagy.Hum Mol Genet. 2012 May 15;21(10):2245-62. doi: 10.1093/hmg/dds040. Epub 2012 Feb 14. Hum Mol Genet. 2012. PMID: 22337954 Free PMC article.
-
Genetic manipulations of mutant huntingtin in mice: new insights into Huntington's disease pathogenesis.FEBS J. 2013 Sep;280(18):4382-94. doi: 10.1111/febs.12418. Epub 2013 Jul 31. FEBS J. 2013. PMID: 23829302 Free PMC article. Review.
-
Role of chaperone-mediated autophagy in degrading Huntington's disease-associated huntingtin protein.Acta Biochim Biophys Sin (Shanghai). 2014 Feb;46(2):83-91. doi: 10.1093/abbs/gmt133. Epub 2013 Dec 8. Acta Biochim Biophys Sin (Shanghai). 2014. PMID: 24323530 Review.
Cited by
-
Insulin and Autophagy in Neurodegeneration.Front Neurosci. 2019 May 22;13:491. doi: 10.3389/fnins.2019.00491. eCollection 2019. Front Neurosci. 2019. PMID: 31231176 Free PMC article. Review.
-
Genetic diversity of axon degenerative mechanisms in models of Parkinson's disease.Neurobiol Dis. 2021 Jul;155:105368. doi: 10.1016/j.nbd.2021.105368. Epub 2021 Apr 20. Neurobiol Dis. 2021. PMID: 33892050 Free PMC article.
-
Chorea-related mutations in PDE10A result in aberrant compartmentalization and functionality of the enzyme.Proc Natl Acad Sci U S A. 2020 Jan 7;117(1):677-688. doi: 10.1073/pnas.1916398117. Epub 2019 Dec 23. Proc Natl Acad Sci U S A. 2020. PMID: 31871190 Free PMC article.
-
Transcription factor EB as a key molecular factor in human health and its implication in diseases.SAGE Open Med. 2023 Feb 28;11:20503121231157209. doi: 10.1177/20503121231157209. eCollection 2023. SAGE Open Med. 2023. PMID: 36891126 Free PMC article. Review.
-
TFEB/Mitf links impaired nuclear import to autophagolysosomal dysfunction in C9-ALS.Elife. 2020 Dec 10;9:e59419. doi: 10.7554/eLife.59419. Elife. 2020. PMID: 33300868 Free PMC article.
References
-
- Cuervo AM. Autophagy: Many paths to the same end. Mol Cell Biochem. 2004;263(1-2):55–72. doi: 10.1023/B:MCBI.0000041848.57020.57 - DOI - PubMed
-
- Klionsky DJ. The molecular machinery of autophagy: Unanswered questions. J Cell Sci. 2005;118(1):7–18. doi: 10.1242/jcs.01620 - DOI - PMC - PubMed
-
- Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4(2):151–75. doi: 10.4161/auto.5338 - DOI - PMC - PubMed
-
- Klionsky DJ, Cuervo AM, Dunn W, Levine B, van der Klei I, Seglen PO. How shall I eat thee? Autophagy. 2007;3(5):413 doi: 10.4161/auto.4377 - DOI - PubMed
-
- Mizushima N. Autophagy: Process and function. Genes Dev. 2007;21(22):2861–73. doi: 10.1101/gad.1599207 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

