Curcumin targets the TFEB-lysosome pathway for induction of autophagy
- PMID: 27689333
- PMCID: PMC5342768
- DOI: 10.18632/oncotarget.12318
Curcumin targets the TFEB-lysosome pathway for induction of autophagy
Abstract
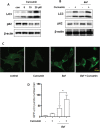
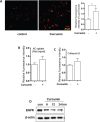
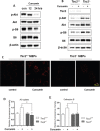
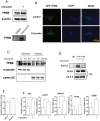
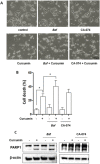
Curcumin is a hydrophobic polyphenol derived from the herb Curcumalonga and its wide spectrum of pharmacological activities has been widely studied. It has been reported that Curcumin can induce autophagy through inhibition of the Akt-mTOR pathway. However, the effect of Curcumin on lysosome remains largely elusive. In this study, we first found that Curcumin treatment enhances autophagic flux in both human colon cancer HCT116 cells and mouse embryonic fibroblasts (MEFs). Moreover, Curcumin treatment promotes lysosomal function, evidenced by the increased lysosomal acidification and enzyme activity. Second, Curcumin is capable of suppressing the mammalian target of rapamycin (mTOR). Interestingly, Curcumin fails to inhibit mTOR and to activate lysosomal function in Tsc2-/-MEFs with constitutive activation of mTOR, indicating that Curcumin-mediated lysosomal activation is achieved via suppression of mTOR. Third, Curcumin treatment activates transcription factor EB (TFEB), a key nuclear transcription factor in control of autophagy and lysosome biogenesis and function, based on the following observations: (i) Curcumin directly binds to TFEB, (ii) Curcumin promotes TFEB nuclear translocation; and (iii) Curcumin increases transcriptional activity of TFEB. Finally, inhibition of autophagy and lysosome leads to more cell death in Curcumin-treated HCT116 cells, suggesting that autophagy and lysosomal activation serves as a cell survival mechanism to protect against Curcumin-mediated cell death. Taken together, data from our study provide a novel insight into the regulatory mechanisms of Curcumin on autophagy and lysosome, which may facilitate the development of Curcumin as a potential cancer therapeutic agent.
Keywords: Curcumin; TFEB; autophagy; lysosome; mTOR.
Conflict of interest statement
The authors confirm that there are no conflicts of interest.
Figures
Similar articles
-
A novel curcumin analog binds to and activates TFEB in vitro and in vivo independent of MTOR inhibition.Autophagy. 2016 Aug 2;12(8):1372-89. doi: 10.1080/15548627.2016.1179404. Epub 2016 May 12. Autophagy. 2016. PMID: 27172265 Free PMC article.
-
The Polyphenolic Compound Curcumin Conjugation with an Alkyne Moiety in the Process of Autophagy.Am J Chin Med. 2018;46(3):673-687. doi: 10.1142/S0192415X18500350. Epub 2018 Apr 4. Am J Chin Med. 2018. PMID: 29614882
-
Cu(II) disrupts autophagy-mediated lysosomal degradation of oligomeric Aβ in microglia via mTOR-TFEB pathway.Toxicol Appl Pharmacol. 2020 Aug 15;401:115090. doi: 10.1016/j.taap.2020.115090. Epub 2020 Jun 5. Toxicol Appl Pharmacol. 2020. PMID: 32512069
-
TFEB at a glance.J Cell Sci. 2016 Jul 1;129(13):2475-81. doi: 10.1242/jcs.146365. Epub 2016 Jun 1. J Cell Sci. 2016. PMID: 27252382 Free PMC article. Review.
-
PRKAA2, MTOR, and TFEB in the regulation of lysosomal damage response and autophagy.J Mol Med (Berl). 2024 Mar;102(3):287-311. doi: 10.1007/s00109-023-02411-7. Epub 2024 Jan 6. J Mol Med (Berl). 2024. PMID: 38183492 Review.
Cited by
-
Curcumin, a Multifaceted Hormetic Agent, Mediates an Intricate Crosstalk between Mitochondrial Turnover, Autophagy, and Apoptosis.Oxid Med Cell Longev. 2020 Jul 18;2020:3656419. doi: 10.1155/2020/3656419. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32765806 Free PMC article. Review.
-
Phytochemicals Bridging Autophagy Induction and Alpha-Synuclein Degradation in Parkinsonism.Int J Mol Sci. 2019 Jul 3;20(13):3274. doi: 10.3390/ijms20133274. Int J Mol Sci. 2019. PMID: 31277285 Free PMC article. Review.
-
Natural Compounds Targeting the Autophagy Pathway in the Treatment of Colorectal Cancer.Int J Mol Sci. 2023 Apr 15;24(8):7310. doi: 10.3390/ijms24087310. Int J Mol Sci. 2023. PMID: 37108476 Free PMC article. Review.
-
Combination Therapy with Nanomicellar-Curcumin and Temozolomide for In Vitro Therapy of Glioblastoma Multiforme via Wnt Signaling Pathways.J Mol Neurosci. 2020 Oct;70(10):1471-1483. doi: 10.1007/s12031-020-01639-z. Epub 2020 Jul 14. J Mol Neurosci. 2020. PMID: 32666415
-
Dietary Polyphenols: A Multifactorial Strategy to Target Alzheimer's Disease.Int J Mol Sci. 2019 Oct 14;20(20):5090. doi: 10.3390/ijms20205090. Int J Mol Sci. 2019. PMID: 31615073 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

