Progression of motor neuron disease is accelerated and the ability to recover is compromised with advanced age in rNLS8 mice
- PMID: 27687289
- PMCID: PMC5043606
- DOI: 10.1186/s40478-016-0377-5
Progression of motor neuron disease is accelerated and the ability to recover is compromised with advanced age in rNLS8 mice
Abstract
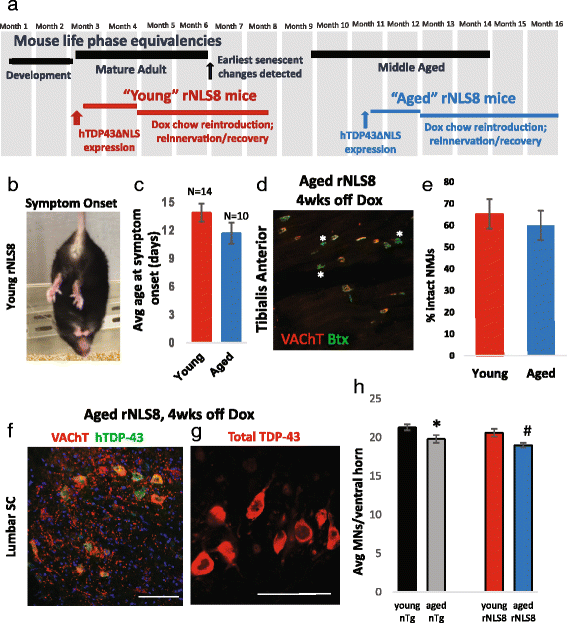
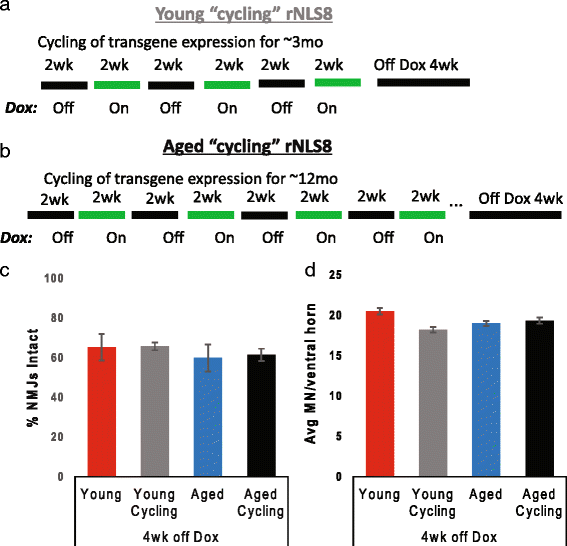
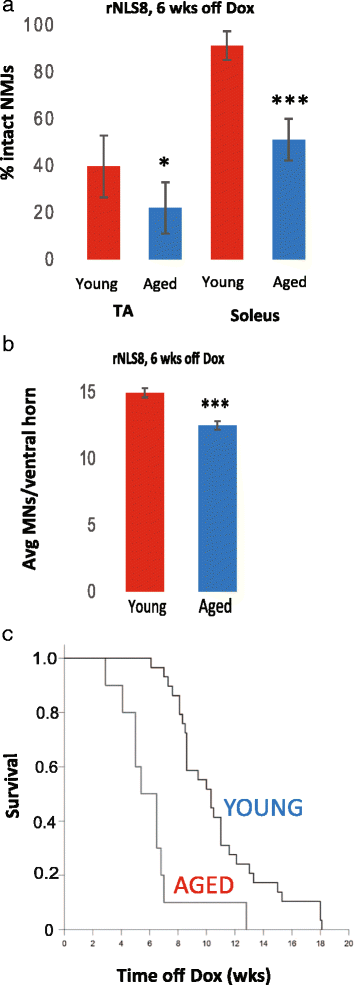
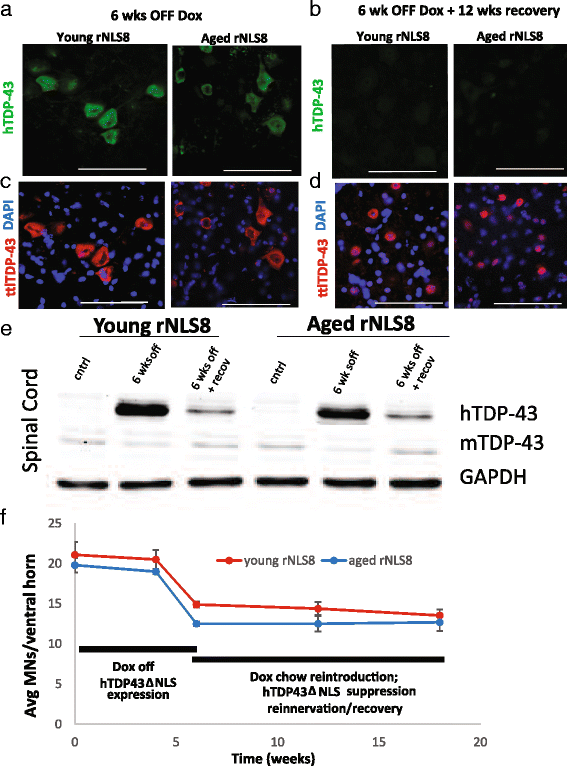
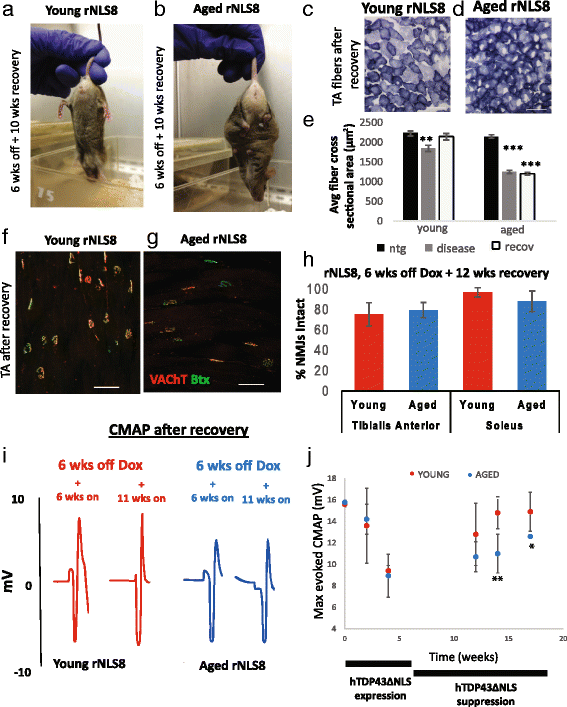
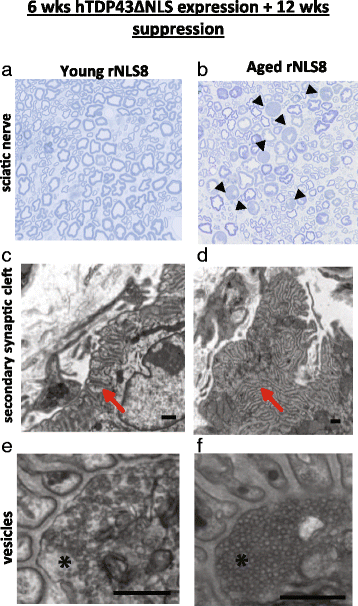
In order to treat progressive paralysis in ALS patients, it is critical to develop a mouse that closely models human ALS in both pathology and also in the timing of these events. We have recently generated new TDP-43 bigenic mice (called rNLS8) with doxycycline (Dox)-suppressible expression of human TDP-43 (hTDP-43) harboring a defective nuclear localization signal (hTDP-43∆NLS) under the control of the NEFH promoter. Our previous studies characterized the pathology and disease course in young rNLS8 mice following induction of neuronal hTDP-43ΔNLS. We now seek to examine if the order and timing of pathologic events are changed in aged mice. We found that the expression of hTDP-43∆NLS in 12+ month old mice did not accelerate the appearance of neuromuscular abnormalities or motor neuron (MN) death in the lumbar spinal cord (SC), though disease progression was accelerated. However, following suppression of the transgene, important differences between young and aged rNLS8 mice emerged in functional motor recovery. We found that recovery was incomplete in aged mice relative to their younger treatment matched counterparts based on gross behavioral measures and physiological recordings from the animals' gastrocnemius (GC) muscles, despite muscle reinnervation by surviving MNs. This is likely because the reinnervation most often only resulted in partial nerve and endplate connections and the muscle's junctional folds were much more disorganized in aged rNLS8 mice. We believe that these studies will be an important basis for the future design and evaluation of therapies designed to slow denervation and promote re-innervation in adult ALS patients.
Keywords: Amyotrophic lateral sclerosis; Motor neuron; Neuromuscular junction; Reinnervation; TDP-43; rNLS mice.
Figures
Similar articles
-
Slow motor neurons resist pathological TDP-43 and mediate motor recovery in the rNLS8 model of amyotrophic lateral sclerosis.Acta Neuropathol Commun. 2022 May 14;10(1):75. doi: 10.1186/s40478-022-01373-0. Acta Neuropathol Commun. 2022. PMID: 35568882 Free PMC article.
-
Functional recovery in new mouse models of ALS/FTLD after clearance of pathological cytoplasmic TDP-43.Acta Neuropathol. 2015 Nov;130(5):643-60. doi: 10.1007/s00401-015-1460-x. Epub 2015 Jul 22. Acta Neuropathol. 2015. PMID: 26197969 Free PMC article.
-
Selective Motor Neuron Resistance and Recovery in a New Inducible Mouse Model of TDP-43 Proteinopathy.J Neurosci. 2016 Jul 20;36(29):7707-17. doi: 10.1523/JNEUROSCI.1457-16.2016. J Neurosci. 2016. PMID: 27445147 Free PMC article.
-
The molecular link between inefficient GluA2 Q/R site-RNA editing and TDP-43 pathology in motor neurons of sporadic amyotrophic lateral sclerosis patients.Brain Res. 2014 Oct 10;1584:28-38. doi: 10.1016/j.brainres.2013.12.011. Epub 2013 Dec 16. Brain Res. 2014. PMID: 24355598 Review.
-
[Molecular link between inefficient GluA2 RNA editing and TDP-43 pathology in ALS motor neurons].Brain Nerve. 2012 May;64(5):549-56. Brain Nerve. 2012. PMID: 22570068 Review. Japanese.
Cited by
-
Reduction of matrix metalloproteinase 9 (MMP-9) protects motor neurons from TDP-43-triggered death in rNLS8 mice.Neurobiol Dis. 2019 Apr;124:133-140. doi: 10.1016/j.nbd.2018.11.013. Epub 2018 Nov 17. Neurobiol Dis. 2019. PMID: 30458231 Free PMC article.
-
TBK1: a new player in ALS linking autophagy and neuroinflammation.Mol Brain. 2017 Feb 2;10(1):5. doi: 10.1186/s13041-017-0287-x. Mol Brain. 2017. PMID: 28148298 Free PMC article. Review.
-
Targeting the glycine-rich domain of TDP-43 with antibodies prevents its aggregation in vitro and reduces neurofilament levels in vivo.Acta Neuropathol Commun. 2023 Jul 11;11(1):112. doi: 10.1186/s40478-023-01592-z. Acta Neuropathol Commun. 2023. PMID: 37434215 Free PMC article.
-
Microglia-mediated recovery from ALS-relevant motor neuron degeneration in a mouse model of TDP-43 proteinopathy.Nat Neurosci. 2018 Mar;21(3):329-340. doi: 10.1038/s41593-018-0083-7. Epub 2018 Feb 20. Nat Neurosci. 2018. PMID: 29463850 Free PMC article.
-
Unsupervised machine learning identifies distinct ALS molecular subtypes in post-mortem motor cortex and blood expression data.Acta Neuropathol Commun. 2023 Dec 21;11(1):208. doi: 10.1186/s40478-023-01686-8. Acta Neuropathol Commun. 2023. PMID: 38129934 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

