Enrichment of hydroxylated C24- and C26-acyl-chain sphingolipids mediates PIN2 apical sorting at trans-Golgi network subdomains
- PMID: 27681606
- PMCID: PMC5056404
- DOI: 10.1038/ncomms12788
Enrichment of hydroxylated C24- and C26-acyl-chain sphingolipids mediates PIN2 apical sorting at trans-Golgi network subdomains
Abstract
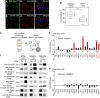
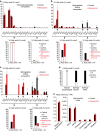
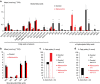
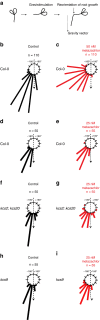
The post-Golgi compartment trans-Golgi Network (TGN) is a central hub divided into multiple subdomains hosting distinct trafficking pathways, including polar delivery to apical membrane. Lipids such as sphingolipids and sterols have been implicated in polar trafficking from the TGN but the underlying mechanisms linking lipid composition to functional polar sorting at TGN subdomains remain unknown. Here we demonstrate that sphingolipids with α-hydroxylated acyl-chains of at least 24 carbon atoms are enriched in secretory vesicle subdomains of the TGN and are critical for de novo polar secretory sorting of the auxin carrier PIN2 to apical membrane of Arabidopsis root epithelial cells. We show that sphingolipid acyl-chain length influences the morphology and interconnections of TGN-associated secretory vesicles. Our results uncover that the sphingolipids acyl-chain length links lipid composition of TGN subdomains with polar secretory trafficking of PIN2 to apical membrane of polarized epithelial cells.
Figures

Similar articles
-
Sphingolipids mediate polar sorting of PIN2 through phosphoinositide consumption at the trans-Golgi network.Nat Commun. 2021 Jul 13;12(1):4267. doi: 10.1038/s41467-021-24548-0. Nat Commun. 2021. PMID: 34257291 Free PMC article.
-
Segregation of sphingolipids and sterols during formation of secretory vesicles at the trans-Golgi network.J Cell Biol. 2009 May 18;185(4):601-12. doi: 10.1083/jcb.200901145. Epub 2009 May 11. J Cell Biol. 2009. PMID: 19433450 Free PMC article.
-
Lipid-dependent protein sorting at the trans-Golgi network.Biochim Biophys Acta. 2012 Aug;1821(8):1059-67. doi: 10.1016/j.bbalip.2011.12.008. Epub 2011 Dec 31. Biochim Biophys Acta. 2012. PMID: 22230596 Review.
-
Trans-Golgi network and subapical compartment of HepG2 cells display different properties in sorting and exiting of sphingolipids.J Biol Chem. 2003 Jan 3;278(1):164-73. doi: 10.1074/jbc.M208259200. Epub 2002 Oct 28. J Biol Chem. 2003. PMID: 12407103
-
Differentiation of Trafficking Pathways at Golgi Entry Core Compartments and Post-Golgi Subdomains.Front Plant Sci. 2020 Dec 8;11:609516. doi: 10.3389/fpls.2020.609516. eCollection 2020. Front Plant Sci. 2020. PMID: 33363561 Free PMC article. Review.
Cited by
-
AtTRAPPC11/ROG2: A Role for TRAPPs in Maintenance of the Plant Trans-Golgi Network/Early Endosome Organization and Function.Plant Cell. 2019 Aug;31(8):1879-1898. doi: 10.1105/tpc.19.00110. Epub 2019 Jun 7. Plant Cell. 2019. PMID: 31175171 Free PMC article.
-
PUCHI regulates very long chain fatty acid biosynthesis during lateral root and callus formation.Proc Natl Acad Sci U S A. 2019 Jul 9;116(28):14325-14330. doi: 10.1073/pnas.1906300116. Epub 2019 Jun 24. Proc Natl Acad Sci U S A. 2019. PMID: 31235573 Free PMC article.
-
A Hybrid Approach Enabling Large-Scale Glycomic Analysis of Post-Golgi Vesicles Reveals a Transport Route for Polysaccharides.Plant Cell. 2019 Mar;31(3):627-644. doi: 10.1105/tpc.18.00854. Epub 2019 Feb 13. Plant Cell. 2019. PMID: 30760563 Free PMC article.
-
Fifteen compelling open questions in plant cell biology.Plant Cell. 2022 Jan 20;34(1):72-102. doi: 10.1093/plcell/koab225. Plant Cell. 2022. PMID: 34529074 Free PMC article. Review.
-
Super resolution live imaging: The key for unveiling the true dynamics of membrane traffic around the Golgi apparatus in plant cells.Front Plant Sci. 2022 Dec 22;13:1100757. doi: 10.3389/fpls.2022.1100757. eCollection 2022. Front Plant Sci. 2022. PMID: 36618665 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

