A Prospective Study of Alemtuzumab as a Second-Line Agent for Steroid-Refractory Acute Graft-versus-Host Disease in Pediatric and Young Adult Allogeneic Hematopoietic Stem Cell Transplantation
- PMID: 27664325
- PMCID: PMC5683393
- DOI: 10.1016/j.bbmt.2016.09.016
A Prospective Study of Alemtuzumab as a Second-Line Agent for Steroid-Refractory Acute Graft-versus-Host Disease in Pediatric and Young Adult Allogeneic Hematopoietic Stem Cell Transplantation
Abstract
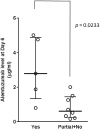
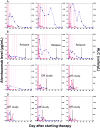
We describe a single-center prospective study of alemtuzumab as a second-line agent for steroid-refractory (SR) acute graft-versus-host disease (aGVHD) in pediatric and young adult allogeneic hematopoietic stem cell transplant recipients. Alemtuzumab was administered for grades II to IV aGVHD if patients did not improve within 5 days or worsened within 48 hours after corticosteroids. Interim analyses of alemtuzumab levels and response were performed after every 5 patients enrolled, resulting in 3 dosing cohorts, as follows: (1) .2 mg/kg alemtuzumab subcutaneously on days 1 to 5 (maximum of 31 mg over 5 days) and .2 mg/kg/dose (not exceeding 10 mg/dose) on days 15, 22, and 29; (2) .2 mg/kg alemtuzumab subcutaneously on days 1 to 5 (maximum of 43 mg over 5 days) and .2 mg/kg/dose on day 7, 10, 15, 22, and 29; and (3) .2 mg/kg subcutaneously on days 1 to 5 and .2 mg/kg/dose on day 7, 10, 15, and 22. Alemtuzumab levels were assessed before starting alemtuzumab and at days 1, 3, 6, 10, and 14 and weekly until day 99, where day 1 was the day of first alemtuzumab dose. Fifteen patients (median age, 10 years; range, 1.4 to 27) received alemtuzumab for grades II (6%), III (74%), and IV (20%) SR-aGVHD. The overall response rate was 67%, with complete response (CR) in 40%, partial response (PR) in 27%, and no response in 33%. The median day 6 alemtuzumab level was 2.79 µg/mL (interquartile range, 1.34 to 4.89) in patients with CR compared with .62 µg/mL (interquartile range, .25 to 1.45) in patients with PR + no response (P < .05). Ninety percent (n = 9) of patients with a CR or PR reduced corticosteroid doses within 8 weeks from first alemtuzumab dose. Side effects included fever (26%) and transient thrombocytopenia (53%). Asymptomatic viremias occurred in all patients but invasive viral disease occurred in 2 patients. One patient developed Epstein-Barr virus-post-transplantation lymphoproliferative disorder. Eighty percent (n = 12) of patients were alive at 6 months, of whom 53% (n = 8) were free of GVHD whereas 13% (n = 2) developed chronic GVHD. Alemtuzumab is an effective second-line agent for children and young adults with SR-aGVHD. Higher alemtuzumab levels are associated with CR. A real-time dose adjusted alemtuzumab study is needed to further optimize the dose of alemtuzumab in aGVHD.
Keywords: Alemtuzumab; Alemtuzumab for GVHD; Alemtuzumab in children; Graft-versus-host disease (GVHD); Steroid-refractory acute graft-versus-host disease (SR-aGVHD).
Copyright © 2016 The American Society for Blood and Marrow Transplantation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
Similar articles
-
[Anti-CD(25) monoclonal antibody as a salvage therapy for steroid-refractory acute graft-versus-host disease in 80 patients receiving allogeneic hematopoietic stem cell transplantation].Zhonghua Nei Ke Za Zhi. 2018 May 1;57(5):324-329. doi: 10.3760/cma.j.issn.0578-1426.2018.05.004. Zhonghua Nei Ke Za Zhi. 2018. PMID: 29747286 Chinese.
-
The successful use of alemtuzumab for treatment of steroid-refractory acute graft-versus-host disease in pediatric patients.Pediatr Transplant. 2014 Feb;18(1):94-102. doi: 10.1111/petr.12183. Epub 2013 Oct 30. Pediatr Transplant. 2014. PMID: 24384050 Free PMC article.
-
Efficacy of Mesenchymal Stem Cell Therapy for Steroid-Refractory Acute Graft-Versus-Host Disease following Allogeneic Hematopoietic Stem Cell Transplantation: A Systematic Review and Meta-Analysis.PLoS One. 2015 Aug 31;10(8):e0136991. doi: 10.1371/journal.pone.0136991. eCollection 2015. PLoS One. 2015. PMID: 26323092 Free PMC article.
-
Alloreactivity as therapeutic principle in the treatment of hematologic malignancies. Studies of clinical and immunologic aspects of allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning.Dan Med Bull. 2007 May;54(2):112-39. Dan Med Bull. 2007. PMID: 17521527 Review.
-
Refractory acute graft-versus-host disease: a new working definition beyond corticosteroid refractoriness.Blood. 2020 Oct 22;136(17):1903-1906. doi: 10.1182/blood.2020007336. Blood. 2020. PMID: 32756949 Review.
Cited by
-
Adverse events in second- and third-line treatments for acute and chronic graft-versus-host disease: systematic review.Ther Adv Hematol. 2020 Dec 8;11:2040620720977039. doi: 10.1177/2040620720977039. eCollection 2020. Ther Adv Hematol. 2020. PMID: 33343855 Free PMC article. Review.
-
Understanding and treatment of cutaneous graft-versus-host-disease.Bone Marrow Transplant. 2023 Dec;58(12):1298-1313. doi: 10.1038/s41409-023-02109-x. Epub 2023 Sep 20. Bone Marrow Transplant. 2023. PMID: 37730800 Free PMC article. Review.
-
A biomarker signature to predict complete response to itacitinib and corticosteroids in acute graft-versus-host disease.Br J Haematol. 2022 Aug;198(4):729-739. doi: 10.1111/bjh.18300. Epub 2022 Jun 11. Br J Haematol. 2022. PMID: 35689489 Free PMC article. Clinical Trial.
-
Current Prophylaxis and Treatment Approaches for Acute Graft-Versus-Host Disease in Haematopoietic Stem Cell Transplantation for Children With Acute Lymphoblastic Leukaemia.Front Pediatr. 2022 Jan 6;9:784377. doi: 10.3389/fped.2021.784377. eCollection 2021. Front Pediatr. 2022. PMID: 35071133 Free PMC article. Review.
-
Decreasing the steroid rapidly may help to improve the clinical outcomes of patients with intestinal steroid-refractory acute graft-versus-host disease receiving basiliximab treatment.Front Oncol. 2024 Mar 26;14:1390438. doi: 10.3389/fonc.2024.1390438. eCollection 2024. Front Oncol. 2024. PMID: 38595816 Free PMC article.
References
-
- Pasquini MC. Impact of graft-versus-host disease on survival. Best Pract Res Clin Haematol. 2008;21:193–204. - PubMed
-
- Couriel D, Saliba R, Hicks K, et al. Tumor necrosis factor-alpha blockade for the treatment of aGVHD. Blood. 2004;104:649–654. - PubMed
-
- Funke VA, de Medeiros CR, Setubal DC, et al. Therapy for severe refractory acute graft-versus-host disease with basiliximab, a selective interleukin-2 receptor antagonist. Bone Marrow Transplant. 2006;37:961–965. - PubMed
-
- Ho VT, Zahrieh D, Hochberg E, et al. Safety and efficacy of denileukin diftitox in patients with steroid-refractory acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Blood. 2004;104:1224–1226. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

