In-silico insights on the prognostic potential of immune cell infiltration patterns in the breast lobular epithelium
- PMID: 27659691
- PMCID: PMC5034260
- DOI: 10.1038/srep33322
In-silico insights on the prognostic potential of immune cell infiltration patterns in the breast lobular epithelium
Abstract
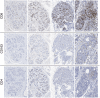
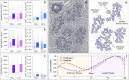
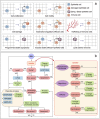
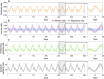
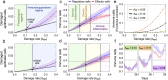
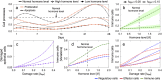
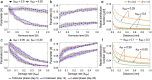
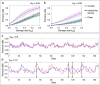
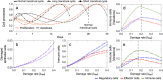
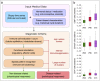
Scattered inflammatory cells are commonly observed in mammary gland tissue, most likely in response to normal cell turnover by proliferation and apoptosis, or as part of immunosurveillance. In contrast, lymphocytic lobulitis (LLO) is a recurrent inflammation pattern, characterized by lymphoid cells infiltrating lobular structures, that has been associated with increased familial breast cancer risk and immune responses to clinically manifest cancer. The mechanisms and pathogenic implications related to the inflammatory microenvironment in breast tissue are still poorly understood. Currently, the definition of inflammation is mainly descriptive, not allowing a clear distinction of LLO from physiological immunological responses and its role in oncogenesis remains unclear. To gain insights into the prognostic potential of inflammation, we developed an agent-based model of immune and epithelial cell interactions in breast lobular epithelium. Physiological parameters were calibrated from breast tissue samples of women who underwent reduction mammoplasty due to orthopedic or cosmetic reasons. The model allowed to investigate the impact of menstrual cycle length and hormone status on inflammatory responses to cell turnover in the breast tissue. Our findings suggested that the immunological context, defined by the immune cell density, functional orientation and spatial distribution, contains prognostic information previously not captured by conventional diagnostic approaches.
Conflict of interest statement
R. Schönmeyer and N. Brieu are employees of Definiens AG. The rest of authors declare no competing financial interests.
Figures


Similar articles
-
Image analysis of immune cell patterns in the human mammary gland during the menstrual cycle refines lymphocytic lobulitis.Breast Cancer Res Treat. 2017 Jul;164(2):305-315. doi: 10.1007/s10549-017-4239-z. Epub 2017 Apr 25. Breast Cancer Res Treat. 2017. PMID: 28444535
-
Exploring the spatial dimension of estrogen and progesterone signaling: detection of nuclear labeling in lobular epithelial cells in normal mammary glands adjacent to breast cancer.Diagn Pathol. 2014;9 Suppl 1(Suppl 1):S11. doi: 10.1186/1746-1596-9-S1-S11. Epub 2014 Dec 19. Diagn Pathol. 2014. PMID: 25565114 Free PMC article.
-
Lobulitis in nonneoplastic breast tissue from breast cancer patients: association with phenotypes that are common in hereditary breast cancer.Hum Pathol. 2014 Jan;45(1):78-84. doi: 10.1016/j.humpath.2013.08.008. Epub 2013 Oct 21. Hum Pathol. 2014. PMID: 24157064
-
Hormones and progeny of breast tumor cells.Climacteric. 2006 Apr;9(2):88-107. doi: 10.1080/13697130600677435. Climacteric. 2006. PMID: 16698656 Review.
-
The fundamental role of mechanical properties in the progression of cancer disease and inflammation.Rep Prog Phys. 2014 Jul;77(7):076602. doi: 10.1088/0034-4885/77/7/076602. Epub 2014 Jul 9. Rep Prog Phys. 2014. PMID: 25006689 Review.
Cited by
-
Hybrid modeling frameworks of tumor development and treatment.Wiley Interdiscip Rev Syst Biol Med. 2020 Jan;12(1):e1461. doi: 10.1002/wsbm.1461. Epub 2019 Jul 17. Wiley Interdiscip Rev Syst Biol Med. 2020. PMID: 31313504 Free PMC article. Review.
-
Integrating mechanism-based T cell phenotypes into a model of tumor-immune cell interactions.APL Bioeng. 2024 Aug 20;8(3):036111. doi: 10.1063/5.0205996. eCollection 2024 Sep. APL Bioeng. 2024. PMID: 39175956 Free PMC article.
-
Integrating digital pathology and mathematical modelling to predict spatial biomarker dynamics in cancer immunotherapy.NPJ Digit Med. 2022 Jul 12;5(1):92. doi: 10.1038/s41746-022-00636-3. NPJ Digit Med. 2022. PMID: 35821064 Free PMC article.
-
Cancer systems immunology.Elife. 2020 Jul 13;9:e53839. doi: 10.7554/eLife.53839. Elife. 2020. PMID: 32657757 Free PMC article. Review.
-
Improving DCIS diagnosis and predictive outcome by applying artificial intelligence.Biochim Biophys Acta Rev Cancer. 2021 Aug;1876(1):188555. doi: 10.1016/j.bbcan.2021.188555. Epub 2021 Apr 29. Biochim Biophys Acta Rev Cancer. 2021. PMID: 33933557 Free PMC article. Review.
References
-
- Andres A.-C. & Strange R. Apoptosis in the estrous and menstrual cycles. Journal of Mammary Gland Biology and Neoplasia 4, 221–228 (1999). - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

