Slow Temporal Integration Enables Robust Neural Coding and Perception of a Cue to Sound Source Location
- PMID: 27656028
- PMCID: PMC5030352
- DOI: 10.1523/JNEUROSCI.1421-16.2016
Slow Temporal Integration Enables Robust Neural Coding and Perception of a Cue to Sound Source Location
Abstract
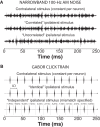
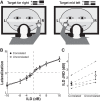
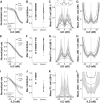
In mammals, localization of sound sources in azimuth depends on sensitivity to interaural differences in sound timing (ITD) and level (ILD). Paradoxically, while typical ILD-sensitive neurons of the auditory brainstem require millisecond synchrony of excitatory and inhibitory inputs for the encoding of ILDs, human and animal behavioral ILD sensitivity is robust to temporal stimulus degradations (e.g., interaural decorrelation due to reverberation), or, in humans, bilateral clinical device processing. Here we demonstrate that behavioral ILD sensitivity is only modestly degraded with even complete decorrelation of left- and right-ear signals, suggesting the existence of a highly integrative ILD-coding mechanism. Correspondingly, we find that a majority of auditory midbrain neurons in the central nucleus of the inferior colliculus (of chinchilla) effectively encode ILDs despite complete decorrelation of left- and right-ear signals. We show that such responses can be accounted for by relatively long windows of bilateral excitatory-inhibitory interaction, which we explicitly measure using trains of narrowband clicks. Neural and behavioral data are compared with the outputs of a simple model of ILD processing with a single free parameter, the duration of excitatory-inhibitory interaction. Behavioral, neural, and modeling data collectively suggest that ILD sensitivity depends on binaural integration of excitation and inhibition within a ≳3 ms temporal window, significantly longer than observed in lower brainstem neurons. This relatively slow integration potentiates a unique role for the ILD system in spatial hearing that may be of particular importance when informative ITD cues are unavailable.
Significance statement: In mammalian hearing, interaural differences in the timing (ITD) and level (ILD) of impinging sounds carry critical information about source location. However, natural sounds are often decorrelated between the ears by reverberation and background noise, degrading the fidelity of both ITD and ILD cues. Here we demonstrate that behavioral ILD sensitivity (in humans) and neural ILD sensitivity (in single neurons of the chinchilla auditory midbrain) remain robust under stimulus conditions that render ITD cues undetectable. This result can be explained by "slow" temporal integration arising from several-millisecond-long windows of excitatory-inhibitory interaction evident in midbrain, but not brainstem, neurons. Such integrative coding can account for the preservation of ILD sensitivity despite even extreme temporal degradations in ecological acoustic stimuli.
Keywords: binaural hearing; interaural decorrelation; interaural level difference; sound localization; temporal integration.
Copyright © 2016 the authors 0270-6474/16/369908-14$15.00/0.
Figures







Similar articles
-
Neural Processing of Acoustic and Electric Interaural Time Differences in Normal-Hearing Gerbils.J Neurosci. 2018 Aug 1;38(31):6949-6966. doi: 10.1523/JNEUROSCI.3328-17.2018. Epub 2018 Jun 29. J Neurosci. 2018. PMID: 29959238 Free PMC article.
-
Sound frequency-invariant neural coding of a frequency-dependent cue to sound source location.J Neurophysiol. 2015 Jul;114(1):531-9. doi: 10.1152/jn.00062.2015. Epub 2015 May 13. J Neurophysiol. 2015. PMID: 25972580 Free PMC article.
-
Stimulus-frequency-dependent dominance of sound localization cues across the cochleotopic map of the inferior colliculus.J Neurophysiol. 2020 May 1;123(5):1791-1807. doi: 10.1152/jn.00713.2019. Epub 2020 Mar 18. J Neurophysiol. 2020. PMID: 32186439 Free PMC article.
-
Neuronal specializations for the processing of interaural difference cues in the chick.Front Neural Circuits. 2014 May 9;8:47. doi: 10.3389/fncir.2014.00047. eCollection 2014. Front Neural Circuits. 2014. PMID: 24847212 Free PMC article. Review.
-
The lateral superior olive: a functional role in sound source localization.Neuroscientist. 2003 Apr;9(2):127-43. doi: 10.1177/1073858403252228. Neuroscientist. 2003. PMID: 12708617 Review.
Cited by
-
These are not the neurons you are looking for.Elife. 2018 Jul 27;7:e39244. doi: 10.7554/eLife.39244. Elife. 2018. PMID: 30052196 Free PMC article.
-
The Calyx of Held: A Hypothesis on the Need for Reliable Timing in an Intensity-Difference Encoder.Neuron. 2018 Nov 7;100(3):534-549. doi: 10.1016/j.neuron.2018.10.026. Neuron. 2018. PMID: 30408442 Free PMC article. Review.
-
Principal cells of the brainstem's interaural sound level detector are temporal differentiators rather than integrators.Elife. 2018 Jun 14;7:e33854. doi: 10.7554/eLife.33854. Elife. 2018. PMID: 29901438 Free PMC article.
-
Robustness of neuronal tuning to binaural sound localization cues against age-related loss of inhibitory synaptic inputs.PLoS Comput Biol. 2021 Jul 9;17(7):e1009130. doi: 10.1371/journal.pcbi.1009130. eCollection 2021 Jul. PLoS Comput Biol. 2021. PMID: 34242210 Free PMC article.
-
Representation of Multidimensional Stimuli: Quantifying the Most Informative Stimulus Dimension from Neural Responses.J Neurosci. 2017 Aug 2;37(31):7332-7346. doi: 10.1523/JNEUROSCI.0318-17.2017. Epub 2017 Jun 29. J Neurosci. 2017. PMID: 28663198 Free PMC article.
References
-
- Boudreau JC, Tsuchitani C. Binaural interaction in the cat superior olive S segment. J Neurophysiol. 1968;31:442–454. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
