RPN13/ADRM1 inhibitor reverses immunosuppression by myeloid-derived suppressor cells
- PMID: 27655678
- PMCID: PMC5340091
- DOI: 10.18632/oncotarget.12095
RPN13/ADRM1 inhibitor reverses immunosuppression by myeloid-derived suppressor cells
Abstract
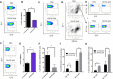
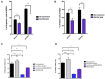
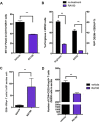
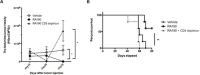
Myeloid-derived-suppressor cells (MDSCs) are key mediators of immune suppression in the ovarian tumor microenvironment. Modulation of MDSC function to relieve immunosuppression may enhance the immunologic clearance of tumors. The bis-benzylidine piperidone RA190 binds to the ubiquitin receptor RPN13/ADRM1 on the 19S regulatory particle of the proteasome and directly kills ovarian cancer cells by triggering proteotoxic stress. Here we examine the effect of RA190 treatment on the immunosuppression induced by MDSCs in the tumor microenvironment, specifically on the immunosuppression induced by MDSCs. We show that RA190 reduces the expression of Stat3 and the levels of key immunosuppressive enzymes and cytokines arginase, iNOS, and IL-10 in MDSCs, while boosting expression of the immunostimulatory cytokine IL-12. Furthermore, we show that the RA190-treated MDSCs lost their capacity to suppress CD8+ T cell function. Finally, we show that RA190 treatment of mice bearing syngeneic ovarian tumor elicits potent CD8+ T cell antitumor immune responses and improves tumor control and survival. These data suggest the potential of RA190 for ovarian cancer treatment by both direct killing of tumor cells via proteasome inhibition and relief of MDSC-mediated suppression of CD8 T cell-dependent antitumor immunity elicited by the apoptotic tumor cells.
Keywords: MDSCs; RPN13; Stat3; immunosuppression; proteasome.
Conflict of interest statement
Under a licensing agreement between Pontifax and the Johns Hopkins University, Drs. Anchoori and Roden are entitled to royalties on an invention described in this article. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies.
Figures


Similar articles
-
Early and consistent overexpression of ADRM1 in ovarian high-grade serous carcinoma.J Ovarian Res. 2017 Aug 7;10(1):53. doi: 10.1186/s13048-017-0347-y. J Ovarian Res. 2017. PMID: 28784174 Free PMC article.
-
Bis-benzylidine Piperidone RA190 treatment of hepatocellular carcinoma via binding RPN13 and inhibiting NF-κB signaling.BMC Cancer. 2020 May 6;20(1):386. doi: 10.1186/s12885-020-06896-0. BMC Cancer. 2020. PMID: 32375699 Free PMC article.
-
Intratumoral injection of mRNA encoding survivin in combination with STAT3 inhibitor stattic enhances antitumor effects.Cancer Lett. 2024 Aug 28;598:217111. doi: 10.1016/j.canlet.2024.217111. Epub 2024 Jul 6. Cancer Lett. 2024. PMID: 38972347
-
Signal transducer and activator of transcription proteins: regulators of myeloid-derived suppressor cell-mediated immunosuppression in cancer.Arch Pharm Res. 2016 Nov;39(11):1597-1608. doi: 10.1007/s12272-016-0822-9. Epub 2016 Aug 29. Arch Pharm Res. 2016. PMID: 27572156 Review.
-
Suppressive role of myeloid-derived suppressor cells (MDSCs) in the microenvironment of breast cancer and targeted immunotherapies.Oncotarget. 2016 Sep 27;7(39):64505-64511. doi: 10.18632/oncotarget.11352. Oncotarget. 2016. PMID: 27542274 Free PMC article. Review.
Cited by
-
Myeloid-Derived Suppressor Cells (MDSCs) in Ovarian Cancer-Looking Back and Forward.Cells. 2023 Jul 22;12(14):1912. doi: 10.3390/cells12141912. Cells. 2023. PMID: 37508575 Free PMC article.
-
Structure-function analyses of candidate small molecule RPN13 inhibitors with antitumor properties.PLoS One. 2020 Jan 15;15(1):e0227727. doi: 10.1371/journal.pone.0227727. eCollection 2020. PLoS One. 2020. PMID: 31940398 Free PMC article.
-
Modulation of Immunosuppression by Oligonucleotide-Based Molecules and Small Molecules Targeting Myeloid-Derived Suppressor Cells.Biomol Ther (Seoul). 2020 Jan 1;28(1):1-17. doi: 10.4062/biomolther.2019.069. Biomol Ther (Seoul). 2020. PMID: 31431006 Free PMC article. Review.
-
Covalent Rpn13-Binding Inhibitors for the Treatment of Ovarian Cancer.ACS Omega. 2018 Sep 30;3(9):11917-11929. doi: 10.1021/acsomega.8b01479. Epub 2018 Sep 27. ACS Omega. 2018. PMID: 30288466 Free PMC article.
-
Drug Development Targeting the Ubiquitin-Proteasome System (UPS) for the Treatment of Human Cancers.Cancers (Basel). 2020 Apr 7;12(4):902. doi: 10.3390/cancers12040902. Cancers (Basel). 2020. PMID: 32272746 Free PMC article. Review.
References
-
- Pitt JM, Marabelle A, Eggermont A, Soria JC, Kroemer G, Zitvogel L. Targeting the tumor microenvironment: removing obstruction to anticancer immune responses and immunotherapy. Ann Oncol. 2016;27:1482–1492. - PubMed
-
- Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, Zanovello P, Segal DM. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol. 2002;168:689–695. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

