The temporally controlled expression of Drongo, the fruit fly homolog of AGFG1, is achieved in female germline cells via P-bodies and its localization requires functional Rab11
- PMID: 27654348
- PMCID: PMC5100350
- DOI: 10.1080/15476286.2016.1218592
The temporally controlled expression of Drongo, the fruit fly homolog of AGFG1, is achieved in female germline cells via P-bodies and its localization requires functional Rab11
Abstract
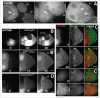
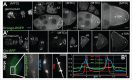
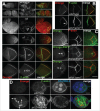
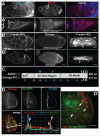
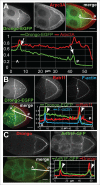
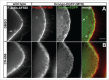
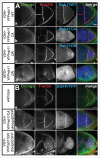
To achieve proper RNA transport and localization, RNA viruses exploit cellular vesicular trafficking pathways. AGFG1, a host protein essential for HIV-1 and Influenza A replication, has been shown to mediate release of intron-containing viral RNAs from the perinuclear region. It is still unknown what its precise role in this release is, or whether AGFG1 also participates in cytoplasmic transport. We report for the first time the expression patterns during oogenesis for Drongo, the fruit fly homolog of AGFG1. We find that temporally controlled Drongo expression is achieved by translational repression of drongo mRNA within P-bodies. Here we show a first link between the recycling endosome pathway and Drongo, and find that proper Drongo localization at the oocyte's cortex during mid-oogenesis requires functional Rab11.
Keywords: AGFG1; Drongo; Drosophila; HIV-1; Influenza A; Rab11; cytoskeleton.
Figures

Similar articles
-
Rab11 maintains connections between germline stem cells and niche cells in the Drosophila ovary.Development. 2007 Oct;134(19):3413-8. doi: 10.1242/dev.008466. Epub 2007 Aug 22. Development. 2007. PMID: 17715175
-
Rab11 polarization of the Drosophila oocyte: a novel link between membrane trafficking, microtubule organization, and oskar mRNA localization and translation.Development. 2002 Jan;129(2):517-26. doi: 10.1242/dev.129.2.517. Development. 2002. PMID: 11807042
-
The KASH domain protein MSP-300 plays an essential role in nuclear anchoring during Drosophila oogenesis.Dev Biol. 2006 Jan 15;289(2):336-45. doi: 10.1016/j.ydbio.2005.10.027. Epub 2005 Dec 7. Dev Biol. 2006. PMID: 16337624
-
Endosomal recycling protein Rab11 in Parkin and Pink1 signaling in Drosophila model of Parkinson's disease.Exp Cell Res. 2022 Nov 15;420(2):113357. doi: 10.1016/j.yexcr.2022.113357. Epub 2022 Sep 15. Exp Cell Res. 2022. PMID: 36116557 Review.
-
Orchestration of cell surface proteins by Rab11.Trends Cell Biol. 2014 Jul;24(7):407-15. doi: 10.1016/j.tcb.2014.02.004. Epub 2014 Mar 24. Trends Cell Biol. 2014. PMID: 24675420 Review.
Cited by
-
PinMol: Python application for designing molecular beacons for live cell imaging of endogenous mRNAs.RNA. 2019 Mar;25(3):305-318. doi: 10.1261/rna.069542.118. Epub 2018 Dec 20. RNA. 2019. PMID: 30573696 Free PMC article.
-
Transcriptome analysis of egg viability in rainbow trout, Oncorhynchus mykiss.BMC Genomics. 2019 Apr 27;20(1):319. doi: 10.1186/s12864-019-5690-5. BMC Genomics. 2019. PMID: 31029084 Free PMC article.
-
A novel likely pathogenic homozygous RBCK1 variant in dilated cardiomyopathy with muscle weakness.ESC Heart Fail. 2024 Jun;11(3):1472-1482. doi: 10.1002/ehf2.14702. Epub 2024 Feb 8. ESC Heart Fail. 2024. PMID: 38329383 Free PMC article.
References
-
- Dong X, Li H, Derdowski A, Ding L, Burnett A, Chen X, Peters TR, Dermody TS, Woodruff E, Wang JJ, et al.. AP-3 directs the intracellular trafficking of HIV-1 Gag and plays a key role in particle assembly. Cell 2005; 120:663-74; PMID:15766529; http://dx.doi.org/10.1016/j.cell.2004.12.023 - DOI - PubMed
-
- Mizuno-Yamasaki E, Rivera-Molina F, Novick P. GTPase networks in membrane traffic. Annu Rev Biochem 2012; 81:637-59; PMID:22463690; http://dx.doi.org/10.1146/annurev-biochem-052810-093700 - DOI - PMC - PubMed
-
- Zhang J, Schulze KL, Hiesinger PR, Suyama K, Wang S, Fish M, Acar M, Hoskins RA, Bellen HJ, Scott MP. Thirty-one flavors of Drosophila Rab proteins. Genetics 2007; 176:1307-22; PMID:17409086; http://dx.doi.org/10.1534/genetics.106.066761 - DOI - PMC - PubMed
-
- Takahashi S, Kubo K, Waguri S, Yabashi A, Shin HW, Katoh Y, Nakayama K. Rab11 regulates exocytosis of recycling vesicles at the plasma membrane. J Cell Sci 2012; 125:4049-57; PMID:22685325; http://dx.doi.org/10.1242/jcs.102913 - DOI - PubMed
-
- Amorim MJ, Bruce EA, Read EK, Foeglein A, Mahen R, Stuart AD, Digard P. A Rab11- and microtubule-dependent mechanism for cytoplasmic transport of influenza A virus viral RNA. J Virol 2011; 85:4143-56; PMID:21307188; http://dx.doi.org/10.1128/JVI.02606-10 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
