Telomerase Regulation from Beginning to the End
- PMID: 27649246
- PMCID: PMC5042394
- DOI: 10.3390/genes7090064
Telomerase Regulation from Beginning to the End
Abstract
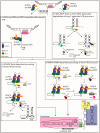
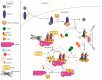
The vast body of literature regarding human telomere maintenance is a true testament to the importance of understanding telomere regulation in both normal and diseased states. In this review, our goal was simple: tell the telomerase story from the biogenesis of its parts to its maturity as a complex and function at its site of action, emphasizing new developments and how they contribute to the foundational knowledge of telomerase and telomere biology.
Keywords: H/ACA ribonucleoprotein; exosome; small Cajal body RNA; small nucleolar RNA; spliceosome; telomerase; telomere.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the writing of the manuscript, and in the decision to publish the manuscript.
Figures
Similar articles
-
The snoRNA domain of vertebrate telomerase RNA functions to localize the RNA within the nucleus.RNA. 2001 Dec;7(12):1833-44. RNA. 2001. PMID: 11780638 Free PMC article.
-
Nucleolar localization of TERT is unrelated to telomerase function in human cells.J Cell Sci. 2008 Jul 1;121(Pt 13):2169-76. doi: 10.1242/jcs.024091. Epub 2008 Jun 3. J Cell Sci. 2008. PMID: 18522991
-
Mouse dyskerin mutations affect accumulation of telomerase RNA and small nucleolar RNA, telomerase activity, and ribosomal RNA processing.Proc Natl Acad Sci U S A. 2004 Jul 20;101(29):10756-61. doi: 10.1073/pnas.0402560101. Epub 2004 Jul 7. Proc Natl Acad Sci U S A. 2004. PMID: 15240872 Free PMC article.
-
Human dyskerin: beyond telomeres.Biol Chem. 2014 Jun;395(6):593-610. doi: 10.1515/hsz-2013-0287. Biol Chem. 2014. PMID: 24468621 Review.
-
Human telomerase: biogenesis, trafficking, recruitment, and activation.Genes Dev. 2015 Jun 1;29(11):1095-105. doi: 10.1101/gad.263863.115. Genes Dev. 2015. PMID: 26063571 Free PMC article. Review.
Cited by
-
Fundamental mechanisms of telomerase action in yeasts and mammals: understanding telomeres and telomerase in cancer cells.Open Biol. 2017 Mar;7(3):160338. doi: 10.1098/rsob.160338. Open Biol. 2017. PMID: 28330934 Free PMC article. Review.
-
Time-Dependent Effects of POT1 Knockdown on Proliferation, Tumorigenicity, and HDACi Response of SK-OV3 Ovarian Cancer Cells.Biomed Res Int. 2018 Feb 6;2018:7184253. doi: 10.1155/2018/7184253. eCollection 2018. Biomed Res Int. 2018. PMID: 29546066 Free PMC article.
-
Study of Telomere Length in Preimplanted Cultured Chondrocytes.Cartilage. 2019 Jan;10(1):36-42. doi: 10.1177/1947603517749918. Epub 2018 Jan 11. Cartilage. 2019. PMID: 29322876 Free PMC article.
-
Inherited human Apollo deficiency causes severe bone marrow failure and developmental defects.Blood. 2022 Apr 21;139(16):2427-2440. doi: 10.1182/blood.2021010791. Blood. 2022. PMID: 35007328 Free PMC article.
-
Berberine Inhibits Telomerase Activity and Induces Cell Cycle Arrest and Telomere Erosion in Colorectal Cancer Cell Line, HCT 116.Molecules. 2021 Jan 13;26(2):376. doi: 10.3390/molecules26020376. Molecules. 2021. PMID: 33450878 Free PMC article.
References
-
- Moyzis R.K., Buckingham J.M., Cram L.S., Dani M., Deaven L.L., Jones M.D., Meyne J., Ratliff R.L., Wu J.R. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc. Natl. Acad. Sci. USA. 1988;85:6622–6626. doi: 10.1073/pnas.85.18.6622. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

