Drug development in Alzheimer's disease: the path to 2025
- PMID: 27646601
- PMCID: PMC5028936
- DOI: 10.1186/s13195-016-0207-9
Drug development in Alzheimer's disease: the path to 2025
Abstract
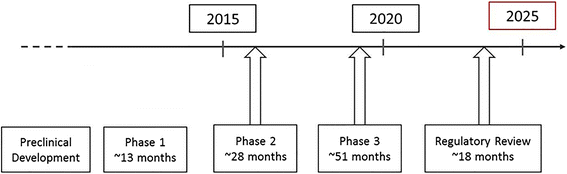
The global impact of Alzheimer's disease (AD) continues to increase, and focused efforts are needed to address this immense public health challenge. National leaders have set a goal to prevent or effectively treat AD by 2025. In this paper, we discuss the path to 2025, and what is feasible in this time frame given the realities and challenges of AD drug development, with a focus on disease-modifying therapies (DMTs). Under the current conditions, only drugs currently in late Phase 1 or later will have a chance of being approved by 2025. If pipeline attrition rates remain high, only a few compounds at best will meet this time frame. There is an opportunity to reduce the time and risk of AD drug development through an improvement in trial design; better trial infrastructure; disease registries of well-characterized participant cohorts to help with more rapid enrollment of appropriate study populations; validated biomarkers to better detect disease, determine risk and monitor disease progression as well as predict disease response; more sensitive clinical assessment tools; and faster regulatory review. To implement change requires efforts to build awareness, educate and foster engagement; increase funding for both basic and clinical research; reduce fragmented environments and systems; increase learning from successes and failures; promote data standardization and increase wider data sharing; understand AD at the basic biology level; and rapidly translate new knowledge into clinical development. Improved mechanistic understanding of disease onset and progression is central to more efficient AD drug development and will lead to improved therapeutic approaches and targets. The opportunity for more than a few new therapies by 2025 is small. Accelerating research and clinical development efforts and bringing DMTs to market sooner would have a significant impact on the future societal burden of AD. As these steps are put in place and plans come to fruition, e.g., approval of a DMT, it can be predicted that momentum will build, the process will be self-sustaining, and the path to 2025, and beyond, becomes clearer.
Keywords: 2025; Alzheimer’s disease; Disease-modifying therapy.
Figures
Similar articles
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Treatment Combinations for Alzheimer's Disease: Current and Future Pharmacotherapy Options.J Alzheimers Dis. 2019;67(3):779-794. doi: 10.3233/JAD-180766. J Alzheimers Dis. 2019. PMID: 30689575 Free PMC article. Review.
-
Clinical Trials for Disease-Modifying Therapies in Alzheimer's Disease: A Primer, Lessons Learned, and a Blueprint for the Future.J Alzheimers Dis. 2018;64(s1):S3-S22. doi: 10.3233/JAD-179901. J Alzheimers Dis. 2018. PMID: 29562511 Free PMC article. Review.
-
Topical Discoveries on Multi-Target Approach to Manage Alzheimer's Disease.Curr Drug Metab. 2018;19(8):704-713. doi: 10.2174/1389200219666180305152553. Curr Drug Metab. 2018. PMID: 29512457 Review.
-
Pharmacotherapy for Alzheimer's disease: what's new on the horizon?Expert Opin Pharmacother. 2022 Aug;23(11):1305-1323. doi: 10.1080/14656566.2022.2097868. Epub 2022 Jul 11. Expert Opin Pharmacother. 2022. PMID: 35793398
Cited by
-
GSK3-ARC/Arg3.1 and GSK3-Wnt signaling axes trigger amyloid-β accumulation and neuroinflammation in middle-aged Shugoshin 1 mice.Aging Cell. 2020 Oct;19(10):e13221. doi: 10.1111/acel.13221. Epub 2020 Aug 28. Aging Cell. 2020. PMID: 32857910 Free PMC article.
-
Dose-response relationship between exercise and cognitive function in older adults with and without cognitive impairment: A systematic review and meta-analysis.PLoS One. 2019 Jan 10;14(1):e0210036. doi: 10.1371/journal.pone.0210036. eCollection 2019. PLoS One. 2019. PMID: 30629631 Free PMC article.
-
Neuron-glia interaction through Serotonin-BDNF-NGFR axis enables regenerative neurogenesis in Alzheimer's model of adult zebrafish brain.PLoS Biol. 2020 Jan 6;18(1):e3000585. doi: 10.1371/journal.pbio.3000585. eCollection 2020 Jan. PLoS Biol. 2020. PMID: 31905199 Free PMC article.
-
Inflammation as a central mechanism in Alzheimer's disease.Alzheimers Dement (N Y). 2018 Sep 6;4:575-590. doi: 10.1016/j.trci.2018.06.014. eCollection 2018. Alzheimers Dement (N Y). 2018. PMID: 30406177 Free PMC article. Review.
-
Selective Serotonin Reuptake Inhibitor-Treatment Does Not Show Beneficial Effects on Cognition or Amyloid Burden in Cognitively Impaired and Cognitively Normal Subjects.Front Aging Neurosci. 2022 Jun 23;14:883256. doi: 10.3389/fnagi.2022.883256. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35813957 Free PMC article.
References
-
- Alzheimer’s Disease International, 2015. https://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf. Accessed 25 May 2016.
-
- National Alzheimer’s Project Act. http://napa.alz.org/national-alzheimers-project-act-backgroun. Accessed 25 May 2016.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials