One-Carbon Metabolism in Health and Disease
- PMID: 27641100
- PMCID: PMC5353360
- DOI: 10.1016/j.cmet.2016.08.009
One-Carbon Metabolism in Health and Disease
Abstract
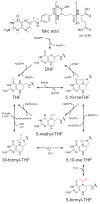
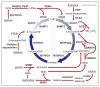
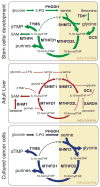
One-carbon (1C) metabolism, mediated by the folate cofactor, supports multiple physiological processes. These include biosynthesis (purines and thymidine), amino acid homeostasis (glycine, serine, and methionine), epigenetic maintenance, and redox defense. Both within eukaryotic cells and across organs, 1C metabolic reactions are compartmentalized. Here we review the fundamentals of mammalian 1C metabolism, including the pathways active in different compartments, cell types, and biological states. Emphasis is given to recent discoveries enabled by modern genetics, analytical chemistry, and isotope tracing. An emerging theme is the biological importance of mitochondrial 1C reactions, both for producing 1C units that are exported to the cytosol and for making additional products, including glycine and NADPH. Increased clarity regarding differential folate pathway usage in cancer, stem cells, development, and adult physiology is reviewed and highlights new opportunities for selective therapeutic intervention.
Keywords: MTHFD2; cancer metabolism; folate; mitochondria; neural tube defects; one-carbon metabolism; serine.
Copyright © 2017. Published by Elsevier Inc.
Figures


Similar articles
-
Therapeutic Targeting of Mitochondrial One-Carbon Metabolism in Cancer.Mol Cancer Ther. 2020 Nov;19(11):2245-2255. doi: 10.1158/1535-7163.MCT-20-0423. Epub 2020 Sep 2. Mol Cancer Ther. 2020. PMID: 32879053 Free PMC article. Review.
-
Tracing Metabolic Fate of Mitochondrial Glycine Cleavage System Derived Formate In Vitro and In Vivo.Int J Mol Sci. 2020 Nov 20;21(22):8808. doi: 10.3390/ijms21228808. Int J Mol Sci. 2020. PMID: 33233834 Free PMC article.
-
Cell cycle regulation of folate-mediated one-carbon metabolism.Wiley Interdiscip Rev Syst Biol Med. 2018 Nov;10(6):e1426. doi: 10.1002/wsbm.1426. Epub 2018 Jun 11. Wiley Interdiscip Rev Syst Biol Med. 2018. PMID: 29889360 Review.
-
Reversal of Cytosolic One-Carbon Flux Compensates for Loss of the Mitochondrial Folate Pathway.Cell Metab. 2016 Jun 14;23(6):1140-1153. doi: 10.1016/j.cmet.2016.04.016. Epub 2016 May 19. Cell Metab. 2016. PMID: 27211901 Free PMC article.
-
The one-carbon metabolism pathway highlights therapeutic targets for gastrointestinal cancer (Review).Int J Oncol. 2017 Apr;50(4):1057-1063. doi: 10.3892/ijo.2017.3885. Epub 2017 Feb 20. Int J Oncol. 2017. PMID: 28259896 Review.
Cited by
-
The folate cycle enzyme MTHFD2 induces cancer immune evasion through PD-L1 up-regulation.Nat Commun. 2021 Mar 29;12(1):1940. doi: 10.1038/s41467-021-22173-5. Nat Commun. 2021. PMID: 33782411 Free PMC article.
-
A happy cell stays home: When metabolic stress creates epigenetic advantages in the tumor microenvironment.Front Oncol. 2022 Aug 26;12:962928. doi: 10.3389/fonc.2022.962928. eCollection 2022. Front Oncol. 2022. PMID: 36091163 Free PMC article. Review.
-
Crosstalk between metabolic reprogramming and epigenetics in cancer: updates on mechanisms and therapeutic opportunities.Cancer Commun (Lond). 2022 Nov;42(11):1049-1082. doi: 10.1002/cac2.12374. Epub 2022 Oct 20. Cancer Commun (Lond). 2022. PMID: 36266736 Free PMC article. Review.
-
Systematic Identification of MACC1-Driven Metabolic Networks in Colorectal Cancer.Cancers (Basel). 2021 Feb 26;13(5):978. doi: 10.3390/cancers13050978. Cancers (Basel). 2021. PMID: 33652667 Free PMC article.
-
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) dysregulates hepatic one carbon metabolism during the progression of steatosis to steatohepatitis with fibrosis in mice.Sci Rep. 2020 Sep 9;10(1):14831. doi: 10.1038/s41598-020-71795-0. Sci Rep. 2020. PMID: 32908189 Free PMC article.
References
-
- Acuna-Hidalgo R, Schanze D, Kariminejad A, Nordgren A, Kariminejad MH, Conner P, Grigelioniene G, Nilsson D, Nordenskjöld M, Wedell A, et al. Neu-Laxova syndrome is a heterogeneous metabolic disorder caused by defects in enzymes of the L-serine biosynthesis pathway. Am J Hum Genet. 2014;95:285–293. - PMC - PubMed
-
- An S, Kumar R, Sheets ED, Benkovic SJ. Reversible compartmentalization of de novo purine biosynthetic complexes in living cells. Science. 2008;320:103–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

