Transmission of a heterologous clade C Symbiodinium in a model anemone infection system via asexual reproduction
- PMID: 27635330
- PMCID: PMC5012276
- DOI: 10.7717/peerj.2358
Transmission of a heterologous clade C Symbiodinium in a model anemone infection system via asexual reproduction
Abstract
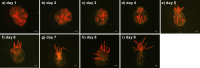
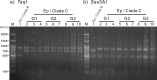
Anemones of genus Exaiptasia are used as model organisms for the study of cnidarian-dinoflagellate (genus Symbiodinium) endosymbiosis. However, while most reef-building corals harbor Symbiodinium of clade C, Exaiptasia spp. anemones mainly harbor clade B Symbiodinium (ITS2 type B1) populations. In this study, we reveal for the first time that bleached Exaiptasia pallida anemones can establish a symbiotic relationship with a clade C Symbiodinium (ITS2 type C1). We further found that anemones can transmit the exogenously supplied clade C Symbiodinium cells to their offspring by asexual reproduction (pedal laceration). In order to corroborate the establishment of stable symbiosis, we used microscopic techniques and genetic analyses to examine several generations of anemones, and the results of these endeavors confirmed the sustainability of the system. These findings provide a framework for understanding the differences in infection dynamics between homologous and heterologous dinoflagellate types using a model anemone infection system.
Keywords: Endosymbiology; Marine biology; Microalgae.
Conflict of interest statement
The authors declare there are no competing interests.
Figures



Similar articles
-
Symbiont Identity Influences Patterns of Symbiosis Establishment, Host Growth, and Asexual Reproduction in a Model Cnidarian-Dinoflagellate Symbiosis.Biol Bull. 2018 Feb;234(1):1-10. doi: 10.1086/696365. Epub 2018 Mar 21. Biol Bull. 2018. PMID: 29694802
-
Establishment of a New Model Sea Anemone for Comparative Studies on Cnidarian-Algal Symbiosis.Zoolog Sci. 2023 Jun;40(3):235-245. doi: 10.2108/zs220099. Zoolog Sci. 2023. PMID: 37256571
-
Population genetic data of a model symbiotic cnidarian system reveal remarkable symbiotic specificity and vectored introductions across ocean basins.Mol Ecol. 2013 Sep;22(17):4499-515. doi: 10.1111/mec.12416. Mol Ecol. 2013. PMID: 23980764
-
Symbiont dynamics during thermal acclimation using cnidarian-dinoflagellate model holobionts.Mar Environ Res. 2017 Sep;130:303-314. doi: 10.1016/j.marenvres.2017.08.005. Epub 2017 Aug 24. Mar Environ Res. 2017. PMID: 28867132
-
Population genetics of reef coral endosymbionts (Symbiodinium, Dinophyceae).Mol Ecol. 2017 May;26(10):2640-2659. doi: 10.1111/mec.14055. Epub 2017 Mar 8. Mol Ecol. 2017. PMID: 28188662 Review.
Cited by
-
Colonization and metabolite profiles of homologous, heterologous and experimentally evolved algal symbionts in the sea anemone Exaiptasia diaphana.ISME Commun. 2022 Mar 30;2(1):30. doi: 10.1038/s43705-022-00114-7. ISME Commun. 2022. PMID: 37938648 Free PMC article.
-
A New Method for Collecting Large Amounts of Symbiotic Gastrodermal Cells from Octocorals.Int J Mol Sci. 2020 May 30;21(11):3911. doi: 10.3390/ijms21113911. Int J Mol Sci. 2020. PMID: 32486176 Free PMC article.
-
Tentacle patterning during Exaiptasia diaphana pedal lacerate development differs between symbiotic and aposymbiotic animals.PeerJ. 2022 Jan 10;10:e12770. doi: 10.7717/peerj.12770. eCollection 2022. PeerJ. 2022. PMID: 35047238 Free PMC article.
-
Biomarker profiling in reef corals of Tonga's Ha'apai and Vava'u archipelagos.PLoS One. 2017 Nov 1;12(11):e0185857. doi: 10.1371/journal.pone.0185857. eCollection 2017. PLoS One. 2017. PMID: 29091723 Free PMC article.
-
A shift away from mutualism under food-deprived conditions in an anemone-dinoflagellate association.PeerJ. 2020 Oct 28;8:e9745. doi: 10.7717/peerj.9745. eCollection 2020. PeerJ. 2020. PMID: 33194344 Free PMC article.
References
-
- Abrego D, Ulstrup KE, Willis BL, Van Oppen MJH. Species–specific interactions between algal endosymbionts and coral hosts define their bleaching response to heat and light stress. Proceedings of the Royal Society B: Biological Sciences. 2008;275:2273–2282. doi: 10.1098/rspb.2008.0180. - DOI - PMC - PubMed
-
- Chen CA, Yang YW, Wei NV, Tsai WS, Fang LS. Symbiont diversity in scleractinian corals from tropical reefs and subtropical non-reef communities in Taiwan. Coral Reefs. 2005;24:11–22. doi: 10.1016/j.bbrc.2004.09.151. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

