MKEY, a Peptide Inhibitor of CXCL4-CCL5 Heterodimer Formation, Protects Against Stroke in Mice
- PMID: 27633389
- PMCID: PMC5079025
- DOI: 10.1161/JAHA.116.003615
MKEY, a Peptide Inhibitor of CXCL4-CCL5 Heterodimer Formation, Protects Against Stroke in Mice
Abstract
Background: MKEY, a synthetic cyclic peptide inhibitor of CXCL4-CCL5 heterodimer formation, has been shown to protect against atherosclerosis and aortic aneurysm formation by mediating inflammation, but whether it modulates neuroinflammation and brain injury has not been studied. We therefore studied the role of MKEY in stroke-induced brain injury in mice.
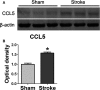
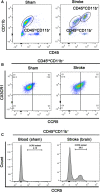
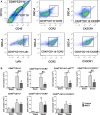
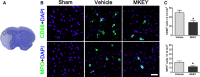
Methods and results: MKEY was injected into mice after stroke with 60 minutes of middle cerebral artery occlusion. Infarct volume and neurological deficit scores were measured. Protein levels of CCL5 and its receptor CCR5 were detected by Western blot and fluorescence-activated cell sorting (FACS), respectively. Numbers of microglia-derived macrophages (MiMΦs) and monocyte-derived MΦs (MoMΦs) in the brain, and their subsets, based on the surface markers CD45, CD11b, CCR2, CX3CR1, and Ly6C, were analyzed by FACS. MΦs and neutrophil infiltration in the ischemic brain were stained with CD68 and myeloperoxidase (MPO), respectively, and assessed by immunofluorescent confocal microscopy. The results showed that expressions of CCL5 and its receptor CCR5, were increased in the ischemic brain after stroke. MKEY injection significantly reduced infarct sizes and improved neurological deficit scores measured 72 hours after stroke. In addition, MKEY injection inhibited the number of MoMΦs, but not MiMΦs, in the ischemic brain. Furthermore, MKEY inhibited protein expression levels of Ly6C,CCR2, and CX3CR1 on MoMΦs. Lastly, the confocal study also suggests that the number of CD68-positive MΦs and MPO-positive neutrophils was inhibited by MKEY injection.
Conclusions: MKEY injection protects against stroke-induced brain injury, probably by inhibiting MoMΦ-mediated neuroinflammation.
Keywords: cerebrovascular disease/stroke; immunology; infarct or infarction; inflammation.
© 2016 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures
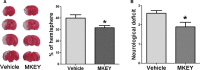
Similar articles
-
Blocking CCL5-CXCL4 heteromerization preserves heart function after myocardial infarction by attenuating leukocyte recruitment and NETosis.Sci Rep. 2018 Jul 13;8(1):10647. doi: 10.1038/s41598-018-29026-0. Sci Rep. 2018. PMID: 30006564 Free PMC article.
-
Peptide inhibitor of CXCL4-CCL5 heterodimer formation, MKEY, inhibits experimental aortic aneurysm initiation and progression.Arterioscler Thromb Vasc Biol. 2013 Apr;33(4):718-26. doi: 10.1161/ATVBAHA.112.300329. Epub 2013 Jan 3. Arterioscler Thromb Vasc Biol. 2013. PMID: 23288157 Free PMC article.
-
C-C Chemokine Receptor Type 5 (CCR5)-Mediated Docking of Transferred Tregs Protects Against Early Blood-Brain Barrier Disruption After Stroke.J Am Heart Assoc. 2017 Aug 2;6(8):e006387. doi: 10.1161/JAHA.117.006387. J Am Heart Assoc. 2017. PMID: 28768648 Free PMC article.
-
Molecular Biology of Atherosclerotic Ischemic Strokes.Int J Mol Sci. 2020 Dec 9;21(24):9372. doi: 10.3390/ijms21249372. Int J Mol Sci. 2020. PMID: 33317034 Free PMC article. Review.
-
Myeloperoxidase: a new target for the treatment of stroke?Neural Regen Res. 2022 Aug;17(8):1711-1716. doi: 10.4103/1673-5374.332130. Neural Regen Res. 2022. PMID: 35017418 Free PMC article. Review.
Cited by
-
Chemokine Heteromers and Their Impact on Cellular Function-A Conceptual Framework.Int J Mol Sci. 2023 Jun 30;24(13):10925. doi: 10.3390/ijms241310925. Int J Mol Sci. 2023. PMID: 37446102 Free PMC article. Review.
-
Rapid Internalization and Nuclear Translocation of CCL5 and CXCL4 in Endothelial Cells.Int J Mol Sci. 2021 Jul 8;22(14):7332. doi: 10.3390/ijms22147332. Int J Mol Sci. 2021. PMID: 34298951 Free PMC article.
-
Monocyte Transmodulation: The Next Novel Therapeutic Approach in Overcoming Ischemic Stroke?Front Neurol. 2020 Oct 22;11:578003. doi: 10.3389/fneur.2020.578003. eCollection 2020. Front Neurol. 2020. PMID: 33193029 Free PMC article. Review.
-
Heterodimers Are an Integral Component of Chemokine Signaling Repertoire.Int J Mol Sci. 2023 Jul 19;24(14):11639. doi: 10.3390/ijms241411639. Int J Mol Sci. 2023. PMID: 37511398 Free PMC article. Review.
-
Blocking CCL5-CXCL4 heteromerization preserves heart function after myocardial infarction by attenuating leukocyte recruitment and NETosis.Sci Rep. 2018 Jul 13;8(1):10647. doi: 10.1038/s41598-018-29026-0. Sci Rep. 2018. PMID: 30006564 Free PMC article.
References
-
- Nabavi SF, Li H, Daglia M, Nabavi SM. Resveratrol and stroke: from chemistry to medicine. Curr Neurovasc Res. 2014;11:390–397. - PubMed
-
- Dziedzic T. Systemic inflammation as a therapeutic target in acute ischemic stroke. Expert Rev Neurother. 2015;15:523–531. - PubMed
-
- Basic Kes V, Simundic AM, Nikolac N, Topic E, Demarin V. Pro‐inflammatory and anti‐inflammatory cytokines in acute ischemic stroke and their relation to early neurological deficit and stroke outcome. Clin Biochem. 2008;41:1330–1334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

