20-Hydroxyeicosatetraenoic Acid (20-HETE) Modulates Canonical Transient Receptor Potential-6 (TRPC6) Channels in Podocytes
- PMID: 27630573
- PMCID: PMC5005377
- DOI: 10.3389/fphys.2016.00351
20-Hydroxyeicosatetraenoic Acid (20-HETE) Modulates Canonical Transient Receptor Potential-6 (TRPC6) Channels in Podocytes
Abstract
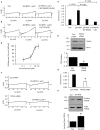
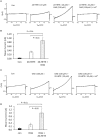
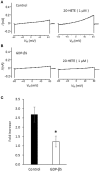
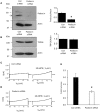
The arachidonic acid metabolite 20-hydroxyeicosatetraenoic acid (20-HETE) regulates renal function, including changes in glomerular function evoked during tubuloglomerular feedback (TGF). This study describes the cellular actions of 20-HETE on cultured podocytes, assessed by whole-cell recordings from cultured podocytes combined with pharmacological and cell-biological manipulations of cells. Bath superfusion of 20-HETE activates cationic currents that are blocked by the pan-TRP blocker SKF-96365 and by 50 μM La(3+), and which are attenuated after siRNA knockdown of TRPC6 subunits. Similar currents are evoked by a membrane-permeable analog of diacylgycerol (OAG), but OAG does not occlude responses to maximally-activating concentrations of 20-HETE (20 μM). Exposure to 20-HETE also increased steady-state surface abundance of TRPC6 subunits in podocytes as assessed by cell-surface biotinylation assays, and increased cytosolic concentrations of reactive oxygen species (ROS). TRPC6 activation by 20-HETE was eliminated in cells pretreated with TEMPOL, a membrane-permeable superoxide dismutase mimic. Activation of TRPC6 by 20-HETE was also blocked when whole-cell recording pipettes contained GDP-βS, indicating a role for either small or heterotrimeric G proteins in the transduction cascade. Responses to 20-HETE were eliminated by siRNA knockdown of podocin, a protein that organizes NADPH oxidase complexes with TRPC6 subunits in this cell type. In summary, modulation of ionic channels in podocytes may contribute to glomerular actions of 20-HETE.
Keywords: FSGS; TRPC6; calcium; eicosanoids; ion channels; podocytes.
Figures

Similar articles
-
NOX2 interacts with podocyte TRPC6 channels and contributes to their activation by diacylglycerol: essential role of podocin in formation of this complex.Am J Physiol Cell Physiol. 2013 Nov 1;305(9):C960-71. doi: 10.1152/ajpcell.00191.2013. Epub 2013 Aug 15. Am J Physiol Cell Physiol. 2013. PMID: 23948707
-
Insulin increases surface expression of TRPC6 channels in podocytes: role of NADPH oxidases and reactive oxygen species.Am J Physiol Renal Physiol. 2012 Feb 1;302(3):F298-307. doi: 10.1152/ajprenal.00423.2011. Epub 2011 Oct 26. Am J Physiol Renal Physiol. 2012. PMID: 22031853 Free PMC article.
-
Angiotensin II activation of TRPC6 channels in rat podocytes requires generation of reactive oxygen species.J Cell Physiol. 2014 Apr;229(4):434-42. doi: 10.1002/jcp.24461. J Cell Physiol. 2014. PMID: 24037962
-
TRPC6 and FSGS: the latest TRP channelopathy.Biochim Biophys Acta. 2007 Aug;1772(8):859-68. doi: 10.1016/j.bbadis.2007.03.005. Epub 2007 Mar 20. Biochim Biophys Acta. 2007. PMID: 17459670 Review.
-
Permeation and Rectification in Canonical Transient Receptor Potential-6 (TRPC6) Channels.Front Physiol. 2018 Aug 3;9:1055. doi: 10.3389/fphys.2018.01055. eCollection 2018. Front Physiol. 2018. PMID: 30123138 Free PMC article. Review.
Cited by
-
Transmembrane insertases and N-glycosylation critically determine synthesis, trafficking, and activity of the nonselective cation channel TRPC6.J Biol Chem. 2019 Aug 23;294(34):12655-12669. doi: 10.1074/jbc.RA119.008299. Epub 2019 Jul 2. J Biol Chem. 2019. PMID: 31266804 Free PMC article.
-
Role of Transient Receptor Potential Canonical Channel 6 (TRPC6) in Diabetic Kidney Disease by Regulating Podocyte Actin Cytoskeleton Rearrangement.J Diabetes Res. 2020 Jan 3;2020:6897390. doi: 10.1155/2020/6897390. eCollection 2020. J Diabetes Res. 2020. PMID: 31998809 Free PMC article. Review.
-
Protective Effects of Low-Dose Alcohol against Acute Stress-Induced Renal Injury in Rats: Involvement of CYP4A/20-HETE and LTB4/BLT1 Pathways.Oxid Med Cell Longev. 2021 Oct 13;2021:4475968. doi: 10.1155/2021/4475968. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34691354 Free PMC article.
-
TRPC channels: Structure, function, regulation and recent advances in small molecular probes.Pharmacol Ther. 2020 May;209:107497. doi: 10.1016/j.pharmthera.2020.107497. Epub 2020 Jan 28. Pharmacol Ther. 2020. PMID: 32004513 Free PMC article. Review.
-
20-HETE synthesis inhibition promotes cerebral protection after intracerebral hemorrhage without inhibiting angiogenesis.J Cereb Blood Flow Metab. 2019 Aug;39(8):1531-1543. doi: 10.1177/0271678X18762645. Epub 2018 Feb 27. J Cereb Blood Flow Metab. 2019. PMID: 29485354 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

