Long-Lived CD4+IFN-γ+ T Cells rather than Short-Lived CD4+IFN-γ+IL-10+ T Cells Initiate Rapid IL-10 Production To Suppress Anamnestic T Cell Responses during Secondary Malaria Infection
- PMID: 27630165
- PMCID: PMC5055201
- DOI: 10.4049/jimmunol.1600968
Long-Lived CD4+IFN-γ+ T Cells rather than Short-Lived CD4+IFN-γ+IL-10+ T Cells Initiate Rapid IL-10 Production To Suppress Anamnestic T Cell Responses during Secondary Malaria Infection
Abstract
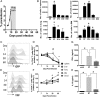
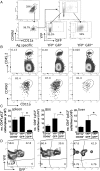
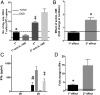
CD4+ T cells that produce IFN-γ are the source of host-protective IL-10 during primary infection with a number of different pathogens, including Plasmodium spp. The fate of these CD4+IFN-γ+IL-10+ T cells following clearance of primary infection and their subsequent influence on the course of repeated infections is, however, presently unknown. In this study, utilizing IFN-γ-yellow fluorescent protein (YFP) and IL-10-GFP dual reporter mice, we show that primary malaria infection-induced CD4+YFP+GFP+ T cells have limited memory potential, do not stably express IL-10, and are disproportionately lost from the Ag-experienced CD4+ T cell memory population during the maintenance phase postinfection. CD4+YFP+GFP+ T cells generally exhibited a short-lived effector rather than effector memory T cell phenotype postinfection and expressed high levels of PD-1, Lag-3, and TIGIT, indicative of cellular exhaustion. Consistently, the surviving CD4+YFP+GFP+ T cell-derived cells were unresponsive and failed to proliferate during the early phase of secondary infection. In contrast, CD4+YFP+GFP- T cell-derived cells expanded rapidly and upregulated IL-10 expression during secondary infection. Correspondingly, CD4+ T cells were the major producers within an accelerated and amplified IL-10 response during the early stage of secondary malaria infection. Notably, IL-10 exerted quantitatively stronger regulatory effects on innate and CD4+ T cell responses during primary and secondary infections, respectively. The results in this study significantly improve our understanding of the durability of IL-10-producing CD4+ T cells postinfection and provide information on how IL-10 may contribute to optimized parasite control and prevention of immune-mediated pathology during repeated malaria infections.
Copyright © 2016 The Authors.
Figures




Similar articles
-
Parasite-Specific CD4+ IFN-γ+ IL-10+ T Cells Distribute within Both Lymphoid and Nonlymphoid Compartments and Are Controlled Systemically by Interleukin-27 and ICOS during Blood-Stage Malaria Infection.Infect Immun. 2015 Oct 12;84(1):34-46. doi: 10.1128/IAI.01100-15. Print 2016 Jan. Infect Immun. 2015. PMID: 26459508 Free PMC article.
-
IL-2 contributes to maintaining a balance between CD4+Foxp3+ regulatory T cells and effector CD4+ T cells required for immune control of blood-stage malaria infection.J Immunol. 2011 Apr 15;186(8):4862-71. doi: 10.4049/jimmunol.1003777. Epub 2011 Mar 9. J Immunol. 2011. PMID: 21389253
-
Effector memory Th1 CD4 T cells are maintained in a mouse model of chronic malaria.PLoS Pathog. 2010 Nov 24;6(11):e1001208. doi: 10.1371/journal.ppat.1001208. PLoS Pathog. 2010. PMID: 21124875 Free PMC article.
-
Priming of CD4+ T cells and development of CD4+ T cell memory; lessons for malaria.Parasite Immunol. 2006 Jan-Feb;28(1-2):25-30. doi: 10.1111/j.1365-3024.2006.00767.x. Parasite Immunol. 2006. PMID: 16438673 Review.
-
The response of CD4+ T cells to Plasmodium chabaudi chabaudi.Immunol Rev. 1989 Dec;112:71-94. doi: 10.1111/j.1600-065x.1989.tb00553.x. Immunol Rev. 1989. PMID: 2575075 Review.
Cited by
-
Expression of TIGIT in splenic and circulatory T cells from mice acutely infected with Toxoplasma gondii.Parasite. 2021;28:13. doi: 10.1051/parasite/2021010. Epub 2021 Feb 25. Parasite. 2021. PMID: 33629951 Free PMC article.
-
Plasmodium vivax Pv12 B-cell epitopes and HLA-DRβ1*-dependent T-cell epitopes in vitro antigenicity.PLoS One. 2018 Sep 10;13(9):e0203715. doi: 10.1371/journal.pone.0203715. eCollection 2018. PLoS One. 2018. PMID: 30199554 Free PMC article.
-
Tim-3 signaling blockade with α-lactose induces compensatory TIGIT expression in Plasmodium berghei ANKA-infected mice.Parasit Vectors. 2019 Nov 11;12(1):534. doi: 10.1186/s13071-019-3788-x. Parasit Vectors. 2019. PMID: 31711531 Free PMC article.
-
What Is Known about the Immune Response Induced by Plasmodium vivax Malaria Vaccine Candidates?Front Immunol. 2017 Feb 13;8:126. doi: 10.3389/fimmu.2017.00126. eCollection 2017. Front Immunol. 2017. PMID: 28243235 Free PMC article. Review.
-
Recent advances on T-cell exhaustion in malaria infection.Med Microbiol Immunol. 2018 Aug;207(3-4):167-174. doi: 10.1007/s00430-018-0547-0. Epub 2018 Jun 23. Med Microbiol Immunol. 2018. PMID: 29936565 Review.
References
-
- Freitas do Rosario A. P., Langhorne J. 2012. T cell-derived IL-10 and its impact on the regulation of host responses during malaria. Int. J. Parasitol. 42: 549–555. - PubMed
-
- Freitas do Rosário A. P., Lamb T., Spence P., Stephens R., Lang A., Roers A., Muller W., O’Garra A., Langhorne J. 2012. IL-27 promotes IL-10 production by effector Th1 CD4+ T cells: a critical mechanism for protection from severe immunopathology during malaria infection. J. Immunol. 188: 1178–1190. - PMC - PubMed
-
- Linke A., Kühn R., Müller W., Honarvar N., Li C., Langhorne J. 1996. Plasmodium chabaudi chabaudi: differential susceptibility of gene-targeted mice deficient in IL-10 to an erythrocytic-stage infection. Exp. Parasitol. 84: 253–263. - PubMed
-
- Couper K. N., Blount D. G., Wilson M. S., Hafalla J. C., Belkaid Y., Kamanaka M., Flavell R. A., de Souza J. B., Riley E. M. 2008. IL-10 from CD4+CD25−Foxp3−CD127− adaptive regulatory T cells modulates parasite clearance and pathology during malaria infection. PLoS Pathog. 4: e1000004. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

