Glucagon-Like Peptide-1 and Its Class B G Protein-Coupled Receptors: A Long March to Therapeutic Successes
- PMID: 27630114
- PMCID: PMC5050443
- DOI: 10.1124/pr.115.011395
Glucagon-Like Peptide-1 and Its Class B G Protein-Coupled Receptors: A Long March to Therapeutic Successes
Abstract
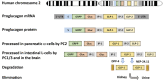
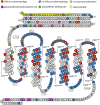
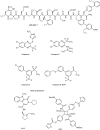
The glucagon-like peptide (GLP)-1 receptor (GLP-1R) is a class B G protein-coupled receptor (GPCR) that mediates the action of GLP-1, a peptide hormone secreted from three major tissues in humans, enteroendocrine L cells in the distal intestine, α cells in the pancreas, and the central nervous system, which exerts important actions useful in the management of type 2 diabetes mellitus and obesity, including glucose homeostasis and regulation of gastric motility and food intake. Peptidic analogs of GLP-1 have been successfully developed with enhanced bioavailability and pharmacological activity. Physiologic and biochemical studies with truncated, chimeric, and mutated peptides and GLP-1R variants, together with ligand-bound crystal structures of the extracellular domain and the first three-dimensional structures of the 7-helical transmembrane domain of class B GPCRs, have provided the basis for a two-domain-binding mechanism of GLP-1 with its cognate receptor. Although efforts in discovering therapeutically viable nonpeptidic GLP-1R agonists have been hampered, small-molecule modulators offer complementary chemical tools to peptide analogs to investigate ligand-directed biased cellular signaling of GLP-1R. The integrated pharmacological and structural information of different GLP-1 analogs and homologous receptors give new insights into the molecular determinants of GLP-1R ligand selectivity and functional activity, thereby providing novel opportunities in the design and development of more efficacious agents to treat metabolic disorders.
Copyright © 2016 by The Author(s).
Figures

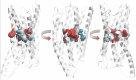
Similar articles
-
The Interplay of Glucagon-Like Peptide-1 Receptor Trafficking and Signalling in Pancreatic Beta Cells.Front Endocrinol (Lausanne). 2021 May 10;12:678055. doi: 10.3389/fendo.2021.678055. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34040588 Free PMC article. Review.
-
Structural Mapping and Functional Characterization of Zebrafish Class B G-Protein Coupled Receptor (GPCR) with Dual Ligand Selectivity towards GLP-1 and Glucagon.PLoS One. 2016 Dec 8;11(12):e0167718. doi: 10.1371/journal.pone.0167718. eCollection 2016. PLoS One. 2016. PMID: 27930690 Free PMC article.
-
Structural Determinants of Binding the Seven-transmembrane Domain of the Glucagon-like Peptide-1 Receptor (GLP-1R).J Biol Chem. 2016 Jun 17;291(25):12991-3004. doi: 10.1074/jbc.M116.721977. Epub 2016 Apr 8. J Biol Chem. 2016. PMID: 27059958 Free PMC article.
-
Truncated Glucagon-like Peptide-1 and Exendin-4 α-Conotoxin pl14a Peptide Chimeras Maintain Potency and α-Helicity and Reveal Interactions Vital for cAMP Signaling in Vitro.J Biol Chem. 2016 Jul 22;291(30):15778-87. doi: 10.1074/jbc.M116.724542. Epub 2016 May 10. J Biol Chem. 2016. PMID: 27226591 Free PMC article.
-
Class B1 GPCRs: insights into multireceptor pharmacology for the treatment of metabolic disease.Am J Physiol Endocrinol Metab. 2024 Nov 1;327(5):E600-E615. doi: 10.1152/ajpendo.00371.2023. Epub 2024 Jul 10. Am J Physiol Endocrinol Metab. 2024. PMID: 38984948 Free PMC article. Review.
Cited by
-
The Interplay of Glucagon-Like Peptide-1 Receptor Trafficking and Signalling in Pancreatic Beta Cells.Front Endocrinol (Lausanne). 2021 May 10;12:678055. doi: 10.3389/fendo.2021.678055. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34040588 Free PMC article. Review.
-
New Developments in Pharmacological Treatment of Obesity and Type 2 Diabetes-Beyond and within GLP-1 Receptor Agonists.Biomedicines. 2024 Jun 13;12(6):1320. doi: 10.3390/biomedicines12061320. Biomedicines. 2024. PMID: 38927527 Free PMC article. Review.
-
Non-peptide agonists and positive allosteric modulators of glucagon-like peptide-1 receptors: Alternative approaches for treatment of Type 2 diabetes.Br J Pharmacol. 2022 Feb;179(4):511-525. doi: 10.1111/bph.15446. Epub 2021 Apr 19. Br J Pharmacol. 2022. PMID: 33724441 Free PMC article. Review.
-
Circulating GLP-1 Levels as a Potential Indicator of Metabolic Syndrome Risk in Adult Women.Nutrients. 2021 Mar 6;13(3):865. doi: 10.3390/nu13030865. Nutrients. 2021. PMID: 33800785 Free PMC article.
-
Kidney Fibrosis and Oxidative Stress: From Molecular Pathways to New Pharmacological Opportunities.Biomolecules. 2024 Jan 22;14(1):137. doi: 10.3390/biom14010137. Biomolecules. 2024. PMID: 38275766 Free PMC article. Review.
References
-
- Abrahamsen N, Lundgren K, Nishimura E. (1995) Regulation of glucagon receptor mRNA in cultured primary rat hepatocytes by glucose and cAMP. J Biol Chem 270:15853–15857. - PubMed
-
- Adelhorst K, Hedegaard BB, Knudsen LB, Kirk O. (1994) Structure-activity studies of glucagon-like peptide-1. J Biol Chem 269:6275–6278. - PubMed
-
- Ahn J-M, Murage EN, Lo S-T, Lin M, and Sun X (2011) Highly constrained GLP-1 analogues as non-invasive PET imaging agents for the assessment of pancreatic beta-cell mass, in Peptides: Building Bridges, Proceedings of the 22nd American Peptide Symposium (Lebl M ed); 2011 June 25-30; San Diego, CA. pp 220-221, American Peptide Society.
-
- Al-Sabah S, Donnelly D. (2003b) The positive charge at Lys-288 of the glucagon-like peptide-1 (GLP-1) receptor is important for binding the N-terminus of peptide agonists. FEBS Lett 553:342–346. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
