Marine compound rhizochalinin shows high in vitro and in vivo efficacy in castration resistant prostate cancer
- PMID: 27626485
- PMCID: PMC5342509
- DOI: 10.18632/oncotarget.11941
Marine compound rhizochalinin shows high in vitro and in vivo efficacy in castration resistant prostate cancer
Abstract
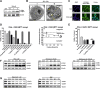
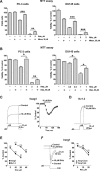
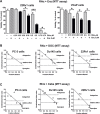
Development of drug resistance is an inevitable phenomenon in castration-resistant prostate cancer (CRPC) cells requiring novel therapeutic approaches. In this study, efficacy and toxicity of Rhizochalinin (Rhiz) - a novel sphingolipid-like marine compound - was evaluated in prostate cancer models, resistant to currently approved standard therapies. In vitro activity and mechanism of action of Rhiz were examined in the human prostate cancer cell lines PC-3, DU145, LNCaP, 22Rv1, and VCaP. Rhiz significantly reduced cell viability at low micromolar concentrations showing most pronounced effects in enzalutamide and abiraterone resistant AR-V7 positive cells. Caspase-dependent apoptosis, inhibition of pro-survival autophagy, downregulation of AR-V7, PSA and IGF-1 expression as well as inhibition of voltage-gated potassium channels were identified as mechanisms of action. Remarkably, Rhiz re-sensitized AR-V7 positive cells to enzalutamide and increased efficacy of taxanes.In vivo activity and toxicity were evaluated in PC-3 and 22Rv1 NOD SCID mouse xenograft models using i.p. administration. Rhiz significantly reduced growth of PC-3 and 22Rv1 tumor xenografts by 27.0% (p = 0.0156) and 46.8% (p = 0.047) compared with controls with an increased fraction of tumor cells showing apoptosis secondary to Rhiz exposure. In line with the in vitro data, Rhiz was most active in AR-V7 positive xenografts in vivo. In animals, no severe side effects were observed.In conclusion, Rhiz is a promising novel marine-derived compound characterized by a unique combination of anticancer properties. Its further clinical development is of high impact for patients suffering from drug resistant prostate cancer especially those harboring AR-V7 mediated resistance to enzalutamide and abiraterone.
Keywords: AR-V7; apoptosis; autophagy; castration resistant prostate cancer; rhizochalinin.
Conflict of interest statement
The authors disclose no potential conflicts of interest.
Figures




Similar articles
-
Androgen Receptor Splice Variant 7 and Efficacy of Taxane Chemotherapy in Patients With Metastatic Castration-Resistant Prostate Cancer.JAMA Oncol. 2015 Aug;1(5):582-91. doi: 10.1001/jamaoncol.2015.1341. JAMA Oncol. 2015. PMID: 26181238 Free PMC article.
-
Proteomic-based investigations on the mode of action of the marine anticancer compound rhizochalinin.Proteomics. 2017 Jun;17(11). doi: 10.1002/pmic.201700048. Proteomics. 2017. PMID: 28445005
-
Recruitment of miR-8080 by luteolin inhibits androgen receptor splice variant 7 expression in castration-resistant prostate cancer.Carcinogenesis. 2020 Aug 12;41(8):1145-1157. doi: 10.1093/carcin/bgz193. Carcinogenesis. 2020. PMID: 31805186 Free PMC article.
-
AR-V7 in circulating tumor cells cluster as a predictive biomarker of abiraterone acetate and enzalutamide treatment in castration-resistant prostate cancer patients.Prostate. 2018 Jun;78(8):576-582. doi: 10.1002/pros.23501. Epub 2018 Mar 5. Prostate. 2018. PMID: 29508425
-
Exploitation of the Androgen Receptor to Overcome Taxane Resistance in Advanced Prostate Cancer.Adv Cancer Res. 2015;127:123-58. doi: 10.1016/bs.acr.2015.03.001. Epub 2015 Mar 29. Adv Cancer Res. 2015. PMID: 26093899 Review.
Cited by
-
Manzamine A reduces androgen receptor transcription and synthesis by blocking E2F8-DNA interactions and effectively inhibits prostate tumor growth in mice.Mol Oncol. 2024 Aug;18(8):1966-1979. doi: 10.1002/1878-0261.13637. Epub 2024 Apr 11. Mol Oncol. 2024. PMID: 38605607 Free PMC article.
-
Marine Natural Products with Activities against Prostate Cancer: Recent Discoveries.Int J Mol Sci. 2023 Jan 11;24(2):1435. doi: 10.3390/ijms24021435. Int J Mol Sci. 2023. PMID: 36674949 Free PMC article. Review.
-
New insights and options into the mechanisms and effects of combined targeted therapy and immunotherapy in prostate cancer.Mol Ther Oncolytics. 2023 Apr 29;29:91-106. doi: 10.1016/j.omto.2023.04.007. eCollection 2023 Jun 15. Mol Ther Oncolytics. 2023. PMID: 37215386 Free PMC article. Review.
-
Synthesis and anticancer activity of the derivatives of marine compound rhizochalin in castration resistant prostate cancer.Oncotarget. 2018 Mar 30;9(24):16962-16973. doi: 10.18632/oncotarget.24764. eCollection 2018 Mar 30. Oncotarget. 2018. PMID: 29682197 Free PMC article.
-
Triptolide Inhibits the AR Signaling Pathway to Suppress the Proliferation of Enzalutamide Resistant Prostate Cancer Cells.Theranostics. 2017 Apr 20;7(7):1914-1927. doi: 10.7150/thno.17852. eCollection 2017. Theranostics. 2017. PMID: 28638477 Free PMC article.
References
-
- Russell PJ, Kingsley EA. Prostate cancer methods and protocols. Springer; New York: 2003. Human prostate cancer cell lines; pp. 21–39. - PubMed
-
- Horwich A, Hugosson J, de Reijke T, Wiegel T, Fizazi K, Kataja V. Prostate cancer: ESMO consensus conference guidelines 2012. Ann Oncol. 2013;24:1141–1162. - PubMed
-
- Caffo O, De Giorgi U, Fratino L, Alesini D, Zagonel V, Facchini G, Gasparro D, Ortega C, Tucci M, Verderame F. Clinical outcomes of castration-resistant prostate cancer treatments administered as third or fourth line following failure of docetaxel and other second-line treatment: results of an Italian multicentre study. Eur Urol. 2015;68:147–153. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

