Glial contributions to visceral pain: implications for disease etiology and the female predominance of persistent pain
- PMID: 27622932
- PMCID: PMC5048206
- DOI: 10.1038/tp.2016.168
Glial contributions to visceral pain: implications for disease etiology and the female predominance of persistent pain
Abstract
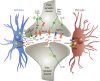
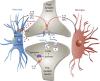
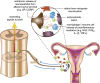
In the central nervous system, bidirectional signaling between glial cells and neurons ('neuroimmune communication') facilitates the development of persistent pain. Spinal glia can contribute to heightened pain states by a prolonged release of neurokine signals that sensitize adjacent centrally projecting neurons. Although many persistent pain conditions are disproportionately common in females, whether specific neuroimmune mechanisms lead to this increased susceptibility remains unclear. This review summarizes the major known contributions of glia and neuroimmune interactions in pain, which has been determined principally in male rodents and in the context of somatic pain conditions. It is then postulated that studying neuroimmune interactions involved in pain attributed to visceral diseases common to females may offer a more suitable avenue for investigating unique mechanisms involved in female pain. Further, we discuss the potential for primed spinal glia and subsequent neurogenic inflammation as a contributing factor in the development of peripheral inflammation, therefore, representing a predisposing factor for females in developing a high percentage of such persistent pain conditions.
Figures
Similar articles
-
The Neuroimmunology of Chronic Pain: From Rodents to Humans.J Neurosci. 2021 Feb 3;41(5):855-865. doi: 10.1523/JNEUROSCI.1650-20.2020. Epub 2020 Nov 25. J Neurosci. 2021. PMID: 33239404 Free PMC article. Review.
-
Role of spinal cord glia in the central processing of peripheral pain perception.Neurogastroenterol Motil. 2010 May;22(5):499-511. doi: 10.1111/j.1365-2982.2010.01491.x. Epub 2010 Mar 16. Neurogastroenterol Motil. 2010. PMID: 20236247 Free PMC article. Review.
-
Neuroimmune Interactions in Pain and Stress: An Interdisciplinary Approach.Neuroscientist. 2021 Apr;27(2):113-128. doi: 10.1177/1073858420914747. Epub 2020 May 22. Neuroscientist. 2021. PMID: 32441204 Free PMC article. Review.
-
Neuroimmune interactions in chronic pain - An interdisciplinary perspective.Brain Behav Immun. 2019 Jul;79:56-62. doi: 10.1016/j.bbi.2019.04.033. Epub 2019 Apr 25. Brain Behav Immun. 2019. PMID: 31029795 Review.
-
Glia and pain: is chronic pain a gliopathy?Pain. 2013 Dec;154 Suppl 1(0 1):S10-S28. doi: 10.1016/j.pain.2013.06.022. Epub 2013 Jun 20. Pain. 2013. PMID: 23792284 Free PMC article. Review.
Cited by
-
From the Bottom-Up: Chemotherapy and Gut-Brain Axis Dysregulation.Front Behav Neurosci. 2018 May 22;12:104. doi: 10.3389/fnbeh.2018.00104. eCollection 2018. Front Behav Neurosci. 2018. PMID: 29872383 Free PMC article. Review.
-
How Microglia Manages Non-cell Autonomous Vicious Cycling of Aβ Toxicity in the Pathogenesis of AD.Front Mol Neurosci. 2020 Nov 17;13:593724. doi: 10.3389/fnmol.2020.593724. eCollection 2020. Front Mol Neurosci. 2020. PMID: 33328884 Free PMC article. Review.
-
The Role of the Thalamus in Nociception: Important but Forgotten.Brain Sci. 2024 Jul 25;14(8):741. doi: 10.3390/brainsci14080741. Brain Sci. 2024. PMID: 39199436 Free PMC article. Review.
-
Mechanistic Insight into the Effects of Curcumin on Neuroinflammation-Driven Chronic Pain.Pharmaceuticals (Basel). 2021 Aug 7;14(8):777. doi: 10.3390/ph14080777. Pharmaceuticals (Basel). 2021. PMID: 34451874 Free PMC article. Review.
-
Anti-Hyperalgesic Efficacy of Acetyl L-Carnitine (ALCAR) Against Visceral Pain Induced by Colitis: Involvement of Glia in the Enteric and Central Nervous System.Int J Mol Sci. 2023 Oct 2;24(19):14841. doi: 10.3390/ijms241914841. Int J Mol Sci. 2023. PMID: 37834289 Free PMC article.
References
-
- King H. Once upon a text: hysteria from Hippocrates. Hippocrates' Woman: Reading the female body in Ancient Greece, 1st edn. Routledge: London, UK, 1998, pp 205–246.
-
- Freud S, Freud A. Observation of a severe case of hemi-anaesthesia in a hysterical male (1886) and Hysteria (1888). The Standard Edition of the Complete Psychological Works of Sigmund Freud: Pre-Psycho-Analytic and Unpublished Drafts. Vintage Classics: London, UK, 2001, pp 23–34, 39–47.
-
- Berkley KJ. Sex differences in pain. Behav Brain Sci 1997; 20: 371–380. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

