Rationale and design of a multicenter randomized controlled study to evaluate the preventive effect of ipragliflozin on carotid atherosclerosis: the PROTECT study
- PMID: 27619983
- PMCID: PMC5020545
- DOI: 10.1186/s12933-016-0449-7
Rationale and design of a multicenter randomized controlled study to evaluate the preventive effect of ipragliflozin on carotid atherosclerosis: the PROTECT study
Abstract
Background: Type 2 diabetes mellitus is associated strongly with an increased risk of micro- and macro-vascular complications, leading to impaired quality of life and shortened life expectancy. In addition to appropriate glycemic control, multi-factorial intervention for a wide range of risk factors, such as hypertension and dyslipidemia, is crucial for management of diabetes. A recent cardiovascular outcome trial in diabetes patients with higher cardiovascular risk demonstrated that a SGLT2 inhibitor markedly reduced mortality, but not macro-vascular events. However, to date there is no clinical evidence regarding the therapeutic effects of SGLT2 inhibitors on arteriosclerosis. The ongoing PROTECT trial was designed to assess whether the SGLT2 inhibitors, ipragliflozin, prevented progression of carotid intima-media thickness in Japanese patients with type 2 diabetes mellitus.
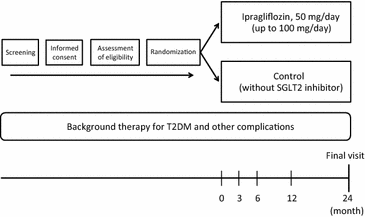
Methods: A total of 480 participants with type 2 diabetes mellitus with a HbA1c between 6 and 10 % despite receiving diet/exercise therapy and/or standard anti-diabetic agents for at least 3 months, will be randomized systematically (1:1) into either ipragliflozin or control (continuation of conventional therapy) groups. After randomization, ipragliflozin (50-100 mg once daily) will be added on to the background therapy in participants assigned to the ipragliflozin group. The primary endpoint of the study is the change in mean intima-media thickness of the common carotid artery from baseline to 24 months. Images of carotid intima-media thickness will be analyzed at a central core laboratory in a blinded manner. The key secondary endpoints include the change from baseline in other parameters of carotid intima-media thickness, various metabolic parameters, and renal function. Other cardiovascular functional tests are also planned for several sub-studies.
Discussion: The PROTECT study is the first to assess the preventive effect of ipragliflozin on progression of carotid atherosclerosis using carotid intima-media thickness as a surrogate marker. The study has potential to clarify the protective effects of ipragliflozin on atherosclerosis. Trial registration Unique Trial Number, JPRN/UMIN000018440 ( https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000021348 ).
Keywords: Atherosclerosis; Intima-media thickness (IMT); Ipragliflozin; SGLT2 inhibitor; Type 2 diabetes mellitus.
Figures
Similar articles
-
Rationale and design of a multicenter placebo-controlled double-blind randomized trial to evaluate the effect of empagliflozin on endothelial function: the EMBLEM trial.Cardiovasc Diabetol. 2017 Apr 12;16(1):48. doi: 10.1186/s12933-017-0532-8. Cardiovasc Diabetol. 2017. PMID: 28403850 Free PMC article. Clinical Trial.
-
Tofogliflozin does not delay progression of carotid atherosclerosis in patients with type 2 diabetes: a prospective, randomized, open-label, parallel-group comparative study.Cardiovasc Diabetol. 2020 Jul 9;19(1):110. doi: 10.1186/s12933-020-01079-4. Cardiovasc Diabetol. 2020. PMID: 32646498 Free PMC article. Clinical Trial.
-
Effect of ipragliflozin on carotid intima-media thickness in patients with type 2 diabetes: a multicenter, randomized, controlled trial.Eur Heart J Cardiovasc Pharmacother. 2023 Feb 2;9(2):165-172. doi: 10.1093/ehjcvp/pvac059. Eur Heart J Cardiovasc Pharmacother. 2023. PMID: 36308299 Free PMC article. Clinical Trial.
-
Profile of Ipragliflozin, an Oral SGLT-2 Inhibitor for the Treatment of Type 2 Diabetes: The Evidence to Date.Drug Des Devel Ther. 2021 Jul 14;15:3057-3069. doi: 10.2147/DDDT.S281602. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 34285473 Free PMC article. Review.
-
Pharmacodynamics, efficacy and safety of sodium-glucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus.Drugs. 2015 Jan;75(1):33-59. doi: 10.1007/s40265-014-0337-y. Drugs. 2015. PMID: 25488697 Review.
Cited by
-
Molecular Mechanisms Underlying the Cardiovascular Benefits of SGLT2i and GLP-1RA.Curr Diab Rep. 2018 Jun 9;18(7):45. doi: 10.1007/s11892-018-1011-7. Curr Diab Rep. 2018. PMID: 29886514 Review.
-
Long-term effects of ipragliflozin on blood pressure in patients with type 2 diabetes: insights from the randomized PROTECT trial.Hypertens Res. 2024 Jan;47(1):168-176. doi: 10.1038/s41440-023-01494-6. Epub 2023 Nov 14. Hypertens Res. 2024. PMID: 37964067 Clinical Trial.
-
Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: a pilot study.Cardiovasc Diabetol. 2017 Oct 23;16(1):138. doi: 10.1186/s12933-017-0621-8. Cardiovasc Diabetol. 2017. PMID: 29061124 Free PMC article.
-
Rationale and design of a multicenter placebo-controlled double-blind randomized trial to evaluate the effect of empagliflozin on endothelial function: the EMBLEM trial.Cardiovasc Diabetol. 2017 Apr 12;16(1):48. doi: 10.1186/s12933-017-0532-8. Cardiovasc Diabetol. 2017. PMID: 28403850 Free PMC article. Clinical Trial.
-
Nonalcoholic Fatty Liver Disease.Handb Exp Pharmacol. 2022;270:233-269. doi: 10.1007/164_2020_352. Handb Exp Pharmacol. 2022. PMID: 32185502 Review.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

