Glomerular basement membrane heparan sulfate in health and disease: A regulator of local complement activation
- PMID: 27609404
- PMCID: PMC5026315
- DOI: 10.1016/j.matbio.2016.09.002
Glomerular basement membrane heparan sulfate in health and disease: A regulator of local complement activation
Abstract
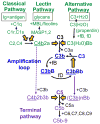
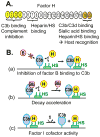
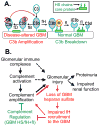
The glomerular basement membrane (GBM) is an essential component of the glomerular filtration barrier. Heparan sulfate proteoglycans such as agrin are major components of the GBM, along with α345(IV) collagen, laminin-521 and nidogen. A loss of GBM heparan sulfate chains is associated with proteinuria in several glomerular diseases and may contribute to the underlying pathology. As the major determinants of the anionic charge of the GBM, heparan sulfate chains have been thought to impart charge selectivity to the glomerular filtration, a view challenged by the negligible albuminuria in mice that lack heparan sulfate in the GBM. Recent studies provide increasing evidence that heparan sulfate chains modulate local complement activation by recruiting complement regulatory protein factor H, the major inhibitor of the alternative pathway in plasma. Factor H selectively inactivates C3b bound to surfaces bearing host-specific polyanions such as heparan sulfate, thus limiting complement activation on self surfaces such as the GBM, which are not protected by cell-bound complement regulators. We discuss mechanisms whereby the acquired loss of GBM heparan sulfate can impair the local regulation of the alternative pathway, exacerbating complement activation and glomerular injury in immune-mediated kidney diseases such as membranous nephropathy and lupus nephritis.
Keywords: Alternative pathway; Complement; Factor H; Glomerular basement membrane; Heparan sulfate.
Copyright © 2016 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Anti-heparan sulfate antibody and functional loss of glomerular heparan sulfate proteoglycans in lupus nephritis.Lupus. 2017 Jul;26(8):815-824. doi: 10.1177/0961203316678674. Epub 2016 Dec 21. Lupus. 2017. PMID: 28420046
-
Glomerular filtration is normal in the absence of both agrin and perlecan-heparan sulfate from the glomerular basement membrane.Nephrol Dial Transplant. 2009 Jul;24(7):2044-51. doi: 10.1093/ndt/gfn758. Epub 2009 Jan 14. Nephrol Dial Transplant. 2009. PMID: 19144998 Free PMC article.
-
The glomerular basement membrane as a barrier to albumin.Nat Rev Nephrol. 2013 Aug;9(8):470-7. doi: 10.1038/nrneph.2013.109. Epub 2013 Jun 18. Nat Rev Nephrol. 2013. PMID: 23774818 Free PMC article. Review.
-
Ultrastructural localization of the three major basement membrane components--type IV collagen, heparan sulfate proteoglycan and laminin--in human membranous glomerulonephritis.Am J Nephrol. 1994;14(1):30-6. doi: 10.1159/000168682. Am J Nephrol. 1994. PMID: 8017478
-
Glomerular heparan sulfate alterations: mechanisms and relevance for proteinuria.Kidney Int. 2000 Feb;57(2):385-400. doi: 10.1046/j.1523-1755.2000.00858.x. Kidney Int. 2000. PMID: 10652015 Review.
Cited by
-
Complexities of the glomerular basement membrane.Nat Rev Nephrol. 2021 Feb;17(2):112-127. doi: 10.1038/s41581-020-0329-y. Epub 2020 Aug 24. Nat Rev Nephrol. 2021. PMID: 32839582 Review.
-
Isotopic Nitrogen-15 Labeling of Mice Identified Long-lived Proteins of the Renal Basement Membranes.Sci Rep. 2020 Mar 24;10(1):5317. doi: 10.1038/s41598-020-62348-6. Sci Rep. 2020. PMID: 32210336 Free PMC article.
-
The Complement System in Metabolic-Associated Kidney Diseases.Front Immunol. 2022 Jul 18;13:902063. doi: 10.3389/fimmu.2022.902063. eCollection 2022. Front Immunol. 2022. PMID: 35924242 Free PMC article. Review.
-
The potential role of complement alternative pathway activation in hypertensive renal damage.Exp Biol Med (Maywood). 2022 May;247(9):797-804. doi: 10.1177/15353702221091986. Epub 2022 Apr 27. Exp Biol Med (Maywood). 2022. PMID: 35473318 Free PMC article.
-
Urinary Protein-Biomarkers Reliably Indicate Very Early Kidney Damage in Children With Alport Syndrome Independently of Albuminuria and Inflammation.Kidney Int Rep. 2023 Sep 29;8(12):2778-2793. doi: 10.1016/j.ekir.2023.09.028. eCollection 2023 Dec. Kidney Int Rep. 2023. PMID: 38106579 Free PMC article.
References
-
- St John PL, Abrahamson DR. Glomerular endothelial cells and podocytes jointly synthesize laminin-1 and -11 chains. Kidney Int. 2001;60(3):1037–46. - PubMed
-
- Barker DF, Hostikka SL, Zhou J, Chow LT, Oliphant AR, Gerken SC, Gregory MC, Skolnick MH, Atkin CL, Tryggvason K. Identification of mutations in the COL4A5 collagen gene in Alport syndrome. Science. 1990;248(4960):1224–7. - PubMed
-
- Mochizuki T, Lemmink HH, Mariyama M, Antignac C, Gubler MC, Pirson Y, Verellen-Dumoulin C, Chan B, Schroder CH, Smeets HJ, et al. Identification of mutations in the alpha 3(IV) and alpha 4(IV) collagen genes in autosomal recessive Alport syndrome. Nature genetics. 1994;8(1):77–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

