Testosterone decreases urinary bladder smooth muscle excitability via novel signaling mechanism involving direct activation of the BK channels
- PMID: 27605581
- PMCID: PMC5210203
- DOI: 10.1152/ajprenal.00238.2016
Testosterone decreases urinary bladder smooth muscle excitability via novel signaling mechanism involving direct activation of the BK channels
Abstract
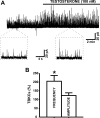
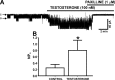
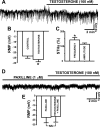
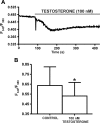
In addition to improving sexual function, testosterone has been reported to have beneficial effects in ameliorating lower urinary tract symptoms by increasing bladder capacity and compliance, while decreasing bladder pressure. However, the cellular mechanisms by which testosterone regulates detrusor smooth muscle (DSM) excitability have not been elucidated. Here, we used amphotericin-B perforated whole cell patch-clamp and single channel recordings on inside-out excised membrane patches to investigate the regulatory role of testosterone in guinea pig DSM excitability. Testosterone (100 nM) significantly increased the depolarization-induced whole cell outward currents in DSM cells. The selective pharmacological inhibition of the large-conductance voltage- and Ca2+-activated K+ (BK) channels with paxilline (1 μM) completely abolished this stimulatory effect of testosterone, suggesting a mechanism involving BK channels. At a holding potential of -20 mV, DSM cells exhibited transient BK currents (TBKCs). Testosterone (100 nM) significantly increased TBKC activity in DSM cells. In current-clamp mode, testosterone (100 nM) significantly hyperpolarized the DSM cell resting membrane potential and increased spontaneous transient hyperpolarizations. Testosterone (100 nM) rapidly increased the single BK channel open probability in inside-out excised membrane patches from DSM cells, clearly suggesting a direct BK channel activation via a nongenomic mechanism. Live-cell Ca2+ imaging showed that testosterone (100 nM) caused a decrease in global intracellular Ca2+ concentration, consistent with testosterone-induced membrane hyperpolarization. In conclusion, the data provide compelling mechanistic evidence that under physiological conditions, testosterone at nanomolar concentrations directly activates BK channels in DSM cells, independent from genomic testosterone receptors, and thus regulates DSM excitability.
Keywords: lower urinary tract symptoms; overactive bladder; testosterone.
Copyright © 2016 the American Physiological Society.
Figures

Similar articles
-
Urinary bladder smooth muscle ion channels: expression, function, and regulation in health and disease.Am J Physiol Renal Physiol. 2020 Aug 1;319(2):F257-F283. doi: 10.1152/ajprenal.00048.2020. Epub 2020 Jul 6. Am J Physiol Renal Physiol. 2020. PMID: 32628539 Free PMC article. Review.
-
Nongenomic modulation of the large conductance voltage- and Ca2+-activated K+ channels by estrogen: A novel regulatory mechanism in human detrusor smooth muscle.Physiol Rep. 2017 Jul;5(14):e13351. doi: 10.14814/phy2.13351. Epub 2017 Jul 27. Physiol Rep. 2017. PMID: 28754781 Free PMC article.
-
Large-conductance voltage- and Ca2+-activated K+ channel regulation by protein kinase C in guinea pig urinary bladder smooth muscle.Am J Physiol Cell Physiol. 2014 Mar 1;306(5):C460-70. doi: 10.1152/ajpcell.00325.2013. Epub 2013 Dec 18. Am J Physiol Cell Physiol. 2014. PMID: 24352333 Free PMC article.
-
Ethanol-mediated relaxation of guinea pig urinary bladder smooth muscle: involvement of BK and L-type Ca2+ channels.Am J Physiol Cell Physiol. 2014 Jan 1;306(1):C45-58. doi: 10.1152/ajpcell.00047.2013. Epub 2013 Oct 23. Am J Physiol Cell Physiol. 2014. PMID: 24153429 Free PMC article.
-
Central role of the BK channel in urinary bladder smooth muscle physiology and pathophysiology.Am J Physiol Regul Integr Comp Physiol. 2014 Sep 15;307(6):R571-84. doi: 10.1152/ajpregu.00142.2014. Epub 2014 Jul 2. Am J Physiol Regul Integr Comp Physiol. 2014. PMID: 24990859 Free PMC article. Review.
Cited by
-
Urinary bladder smooth muscle ion channels: expression, function, and regulation in health and disease.Am J Physiol Renal Physiol. 2020 Aug 1;319(2):F257-F283. doi: 10.1152/ajprenal.00048.2020. Epub 2020 Jul 6. Am J Physiol Renal Physiol. 2020. PMID: 32628539 Free PMC article. Review.
-
Associations between exposure to organophosphate esters and overactive bladder in U.S. adults: a cross-sectional study.Front Public Health. 2023 Nov 9;11:1186848. doi: 10.3389/fpubh.2023.1186848. eCollection 2023. Front Public Health. 2023. PMID: 38026372 Free PMC article.
-
Blockade of Acid-Sensing Ion Channels Increases Urinary Bladder Capacity With or Without Intravesical Irritation in Mice.Front Physiol. 2020 Oct 26;11:592867. doi: 10.3389/fphys.2020.592867. eCollection 2020. Front Physiol. 2020. PMID: 33192609 Free PMC article.
-
Cohesive cancer invasion of the biophysical barrier of smooth muscle.Cancer Metastasis Rev. 2021 Mar;40(1):205-219. doi: 10.1007/s10555-020-09950-2. Epub 2021 Jan 4. Cancer Metastasis Rev. 2021. PMID: 33398621 Free PMC article. Review.
-
Impact of Testosterone Deficiency and Testosterone Therapy on Lower Urinary Tract Symptoms in Men with Metabolic Syndrome.World J Mens Health. 2018 Sep;36(3):199-222. doi: 10.5534/wjmh.180032. Epub 2018 Jul 3. World J Mens Health. 2018. PMID: 30079638 Free PMC article. Review.
References
-
- Abdel-Hamid AAM, Ali EMT. Effect of testosterone therapy on the urinary bladder in experimental hypogonadism of rats. J Mol Histol 46: 263–272, 2015. - PubMed
-
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, Van Kerrebroeck P, Victor A, Wein A. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology 61: 37–49, 2003. - PubMed
-
- Cairrao E, Alvarez E, Santos-Silva AJ, Verde I. Potassium channels are involved in testosterone-induced vasorelaxation of human umbilical artery. Naunyn Schmiedebergs Arch Pharmacol 376: 375–383, 2008. - PubMed
-
- Coyne KS, Kaplan SA, Chapple CR, Sexton CC, Kopp ZS, Bush EN, Aiyer LP. Risk factors and comorbid conditions associated with lower urinary tract symptoms: EpiLUTS. BJU Int 103, Suppl 3: 24–32, 2009. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

