Microbiota-myeloid cell crosstalk beyond the gut
- PMID: 27605211
- PMCID: PMC6608064
- DOI: 10.1189/jlb.3RI0516-222R
Microbiota-myeloid cell crosstalk beyond the gut
Abstract
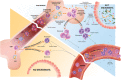
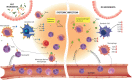
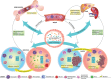
The gut microbiota is a complex and dynamic microbial ecosystem that plays a fundamental role in host physiology. Locally, the gut commensal microbes/host symbiotic relationship is vital for barrier fortification, nutrient absorption, resistance against intestinal pathogens, and the development and maintenance of the mucosal immune system. It is now clear that the effects of the indigenous intestinal flora extend beyond the gut, ranging from shaping systemic immune responses to metabolic and behavioral functions. However, the underlying mechanisms of the gut microbiota/systemic immune system interactions remain largely unknown. Myeloid cells respond to microbial signals, including those derived from commensals, and initiate innate and adaptive immune responses. In this review, we focus on the impact of the gut microbiota on myeloid cells at extraintestinal sites. In particular, we discuss how commensal-derived signals affect steady-state myelopoiesis and cellular function and how that influences the response to infection and cancer therapy.
Keywords: cancer; host–microbial interactions; infection; mononuclear phagocytes; systemic immunity.
© Society for Leukocyte Biology.
Figures

Similar articles
-
The Roles of Inflammation, Nutrient Availability and the Commensal Microbiota in Enteric Pathogen Infection.Microbiol Spectr. 2015 Jun;3(3). doi: 10.1128/microbiolspec.MBP-0008-2014. Microbiol Spectr. 2015. PMID: 26185088
-
Bridging intestinal immunity and gut microbiota by metabolites.Cell Mol Life Sci. 2019 Oct;76(20):3917-3937. doi: 10.1007/s00018-019-03190-6. Epub 2019 Jun 27. Cell Mol Life Sci. 2019. PMID: 31250035 Free PMC article. Review.
-
Role of intestinal microbiota and metabolites on gut homeostasis and human diseases.BMC Immunol. 2017 Jan 6;18(1):2. doi: 10.1186/s12865-016-0187-3. BMC Immunol. 2017. PMID: 28061847 Free PMC article. Review.
-
Connecting the immune system, systemic chronic inflammation and the gut microbiome: The role of sex.J Autoimmun. 2018 Aug;92:12-34. doi: 10.1016/j.jaut.2018.05.008. Epub 2018 Jun 1. J Autoimmun. 2018. PMID: 29861127 Review.
-
Gut Microbiota Modulation on Intestinal Mucosal Adaptive Immunity.J Immunol Res. 2019 Oct 3;2019:4735040. doi: 10.1155/2019/4735040. eCollection 2019. J Immunol Res. 2019. PMID: 31687412 Free PMC article. Review.
Cited by
-
Silent neonatal influenza A virus infection primes systemic antimicrobial immunity.Front Immunol. 2023 Jan 24;14:1072142. doi: 10.3389/fimmu.2023.1072142. eCollection 2023. Front Immunol. 2023. PMID: 36761727 Free PMC article.
-
Potential Role of Gut Microbiota in Induction and Regulation of Innate Immune Memory.Front Immunol. 2019 Oct 25;10:2441. doi: 10.3389/fimmu.2019.02441. eCollection 2019. Front Immunol. 2019. PMID: 31749793 Free PMC article. Review.
-
The Effect of the Gut Microbiota on Systemic and Anti-Tumor Immunity and Response to Systemic Therapy against Cancer.Cancers (Basel). 2022 Jul 22;14(15):3563. doi: 10.3390/cancers14153563. Cancers (Basel). 2022. PMID: 35892821 Free PMC article. Review.
-
Protective Microbiota: From Localized to Long-Reaching Co-Immunity.Front Immunol. 2017 Dec 7;8:1678. doi: 10.3389/fimmu.2017.01678. eCollection 2017. Front Immunol. 2017. PMID: 29270167 Free PMC article. Review.
-
[Correlation between Gut Microbiota and Lung Cancer].Zhongguo Fei Ai Za Zhi. 2020 Oct 20;23(10):909-915. doi: 10.3779/j.issn.1009-3419.2020.101.39. Epub 2020 Aug 17. Zhongguo Fei Ai Za Zhi. 2020. PMID: 32798442 Free PMC article. Review. Chinese.
References
-
- McFall‐Ngai, M. , Hadfield, M. G. , Bosch, T. C. , Carey, H. V. , Domazet‐Losno, T. , Douglas, A. E. , Dubilier, N. , Eberl, G. , Fukami, T. , Gilbert, S. F. , Hentschel, U. , King, N. , Kjelleberg, S. , Knoll, A. H. , Kremer, N. , Mazmanian, S. K. , Metcalf, J. L. , Nealson, K. , Pierce, N. E. , Rawls, J. F. , Reid, A. , Ruby, E. G. , Rumpho, M. , Sanders, J. G. , Tautz, D. , Wernegreen, J. J. (2013) Animals in a bacterial world, a new imperative for the life sciences. Proc. Natl. Acad. Sci. USA 110, 3229–3236. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

