A Partial E3 Deletion in Replication-Defective Adenoviral Vectors Allows for Stable Expression of Potentially Toxic Transgene Products
- PMID: 27604324
- PMCID: PMC5069709
- DOI: 10.1089/hgtb.2016.044
A Partial E3 Deletion in Replication-Defective Adenoviral Vectors Allows for Stable Expression of Potentially Toxic Transgene Products
Erratum in
-
Correction to: Hum Gene Ther Methods 2016;27(5):187-196.Hum Gene Ther Methods. 2017 Feb;28(1):60. doi: 10.1089/hgtb.2016.044.correx. Epub 2017 Jan 31. Hum Gene Ther Methods. 2017. PMID: 28140663 Free PMC article. No abstract available.
Abstract
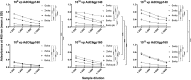
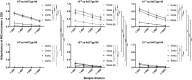
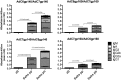
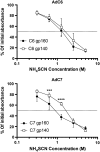
Adenovirus (Ad) is used extensively for construction of viral vectors, most commonly with deletion in its E1 and/or E3 genomic regions. Previously, our attempts to insert envelope proteins (Env) of HIV-1 into such vectors based on chimpanzee-derived Ad (AdC) viruses were thwarted. Here, we describe that genetic instability of an E1- and E3-deleted AdC vector of serotype C6 expressing Env of HIV-1 can be overcome by reinsertion of E3 sequences with anti-apoptotic activities. This partial E3 deletion presumably delays premature death of HEK-293 packaging cell lines due to Env-induced cell apoptosis. The same partial E3 deletion also allows for the generation of stable glycoprotein 140 (gp140)- and gp160-expressing Ad vectors based on AdC7, a distinct AdC serotype. Env-expressing AdC vectors containing the partial E3 deletion are genetically stable upon serial cell culture passaging, produce yields comparable to those of other AdC vectors, and induce transgene product-specific antibody responses in mice. A partial E3 deletion thereby allows expansion of the repertoire of transgenes that can be expressed by Ad vectors.
Keywords: HIV-1; genetic stability; immune responses; vaccine.
Conflict of interest statement
Author Disclosure No competing financial interests exist.
Figures


Similar articles
-
Effects of the deletion of early region 4 (E4) open reading frame 1 (orf1), orf1-2, orf1-3 and orf1-4 on virus-host cell interaction, transgene expression, and immunogenicity of replicating adenovirus HIV vaccine vectors.PLoS One. 2013 Oct 15;8(10):e76344. doi: 10.1371/journal.pone.0076344. eCollection 2013. PLoS One. 2013. PMID: 24143187 Free PMC article.
-
Construction and characterization of E1- and E3-deleted adenovirus vectors expressing two antigens from two separate expression cassettes.Hum Gene Ther. 2014 Apr;25(4):328-38. doi: 10.1089/hum.2013.216. Epub 2014 Mar 25. Hum Gene Ther. 2014. PMID: 24367921 Free PMC article.
-
Attenuation of Replication-Competent Adenovirus Serotype 26 Vaccines by Vectorization.Clin Vaccine Immunol. 2015 Nov;22(11):1166-75. doi: 10.1128/CVI.00510-15. Epub 2015 Sep 16. Clin Vaccine Immunol. 2015. PMID: 26376928 Free PMC article.
-
Adenovirus vectors deleted for genes essential for viral DNA replication.Front Biosci. 2005 May 1;10:1146-55. doi: 10.2741/1607. Front Biosci. 2005. PMID: 15769613 Review.
-
The ups and downs of adenovirus vectors.Bull N Y Acad Med. 1996 Summer;73(1):53-8. Bull N Y Acad Med. 1996. PMID: 8804738 Free PMC article. Review.
Cited by
-
Generation and Characterization of a Replication-Competent Human Adenovirus Type 55 Encoding EGFP.Viruses. 2023 May 18;15(5):1192. doi: 10.3390/v15051192. Viruses. 2023. PMID: 37243276 Free PMC article.
-
Porcine Adenovirus Type 3 E3 Encodes a Structural Protein Essential for Capsid Stability and Production of Infectious Progeny Virions.J Virol. 2018 Sep 26;92(20):e00680-18. doi: 10.1128/JVI.00680-18. Print 2018 Oct 15. J Virol. 2018. PMID: 30068639 Free PMC article.
References
-
- Benihoud K, Yeh P, Perricaudet M. Adenovirus vectors for gene delivery. Curr Opin Biotechnol 1999;10:440–447 - PubMed
-
- Appaiahgari MB, Vrati S. Adenoviruses as gene/vaccine delivery vectors: promises and pitfalls. Expert Opin Biol Ther 2015;15:337–351 - PubMed
-
- Muruve DA. The innate immune response to adenovirus vectors. Hum Gene Ther 2004;15:1157–66 - PubMed
-
- Hensley SE, Giles-Davis W, McCoy KC, et al. . Dendritic cell maturation, but not CD8+ T cell induction, is dependent on type I IFN signaling during vaccination with adenovirus vectors. J Immunol 2005;175:6032–6041 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

