Engineered Breast Cancer Cell Spheroids Reproduce Biologic Properties of Solid Tumors
- PMID: 27603912
- PMCID: PMC5142748
- DOI: 10.1002/adhm.201600644
Engineered Breast Cancer Cell Spheroids Reproduce Biologic Properties of Solid Tumors
Abstract
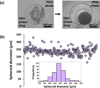
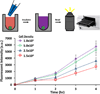
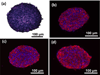
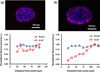
Solid tumors develop as 3D tissue constructs. As tumors grow larger, spatial gradients of nutrients and oxygen and inadequate diffusive supply to cells distant from vasculature develops. Hypoxia initiates signaling and transcriptional alterations to promote survival of cancer cells and generation of cancer stem cells (CSCs) that have self-renewal and tumor-initiation capabilities. Both hypoxia and CSCs are associated with resistance to therapies and tumor relapse. This study demonstrates that 3D cancer cell models, known as tumor spheroids, generated with a polymeric aqueous two-phase system (ATPS) technology capture these important biological processes. Similar to solid tumors, spheroids of triple negative breast cancer cells deposit major extracellular matrix proteins. The molecular analysis establishes presence of hypoxic cells in the core region and expression of CSC gene and protein markers including CD24, CD133, and Nanog. Importantly, these spheroids resist treatment with chemotherapy drugs. A combination treatment approach using a hypoxia-activated prodrug, TH-302, and a chemotherapy drug, doxorubicin, successfully targets drug resistant spheroids. This study demonstrates that ATPS spheroids recapitulate important biological and functional properties of solid tumors and provide a unique model for studies in cancer research.
Keywords: aqueous two-phase systems; cancer stem cells; drug resistance; hypoxia; spheroids.
© 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


Similar articles
-
Standard chemotherapy impacts on in vitro cellular heterogeneity in spheroids enriched with cancer stem cells (CSCs) derived from triple-negative breast cancer cell line.Biochem Biophys Res Commun. 2024 Nov 19;734:150765. doi: 10.1016/j.bbrc.2024.150765. Epub 2024 Sep 30. Biochem Biophys Res Commun. 2024. PMID: 39357337
-
Development and characterization of a human three-dimensional chondrosarcoma culture for in vitro drug testing.PLoS One. 2017 Jul 13;12(7):e0181340. doi: 10.1371/journal.pone.0181340. eCollection 2017. PLoS One. 2017. PMID: 28704566 Free PMC article.
-
Molecular and cellular pharmacology of the hypoxia-activated prodrug TH-302.Mol Cancer Ther. 2012 Mar;11(3):740-51. doi: 10.1158/1535-7163.MCT-11-0634. Epub 2011 Dec 6. Mol Cancer Ther. 2012. PMID: 22147748
-
3D tumor spheroids as in vitro models to mimic in vivo human solid tumors resistance to therapeutic drugs.Biotechnol Bioeng. 2019 Jan;116(1):206-226. doi: 10.1002/bit.26845. Epub 2018 Oct 27. Biotechnol Bioeng. 2019. PMID: 30367820 Review.
-
Evofosfamide, a new horizon in the treatment of pancreatic cancer.Anticancer Drugs. 2016 Sep;27(8):723-5. doi: 10.1097/CAD.0000000000000386. Anticancer Drugs. 2016. PMID: 27232101 Review.
Cited by
-
Three-Dimensional Tumor Models to Study Cancer Stemness-Mediated Drug Resistance.Cell Mol Bioeng. 2024 Feb 21;17(2):107-119. doi: 10.1007/s12195-024-00798-y. eCollection 2024 Apr. Cell Mol Bioeng. 2024. PMID: 38737455 Free PMC article. Review.
-
Synergistic Inhibition of Kinase Pathways Overcomes Resistance of Colorectal Cancer Spheroids to Cyclic Targeted Therapies.ACS Pharmacol Transl Sci. 2019 Jul 19;2(4):275-284. doi: 10.1021/acsptsci.9b00042. eCollection 2019 Aug 9. ACS Pharmacol Transl Sci. 2019. PMID: 32259061 Free PMC article.
-
Microchamber Cultures of Bladder Cancer: A Platform for Characterizing Drug Responsiveness and Resistance in PDX and Primary Cancer Cells.Sci Rep. 2017 Sep 25;7(1):12277. doi: 10.1038/s41598-017-12543-9. Sci Rep. 2017. PMID: 28947782 Free PMC article.
-
Biomaterials-Based Approaches to Tumor Spheroid and Organoid Modeling.Adv Healthc Mater. 2018 Mar;7(6):e1700980. doi: 10.1002/adhm.201700980. Epub 2017 Dec 4. Adv Healthc Mater. 2018. PMID: 29205942 Free PMC article. Review.
-
Optical tissue clearing associated with 3D imaging: application in preclinical and clinical studies.Histochem Cell Biol. 2022 May;157(5):497-511. doi: 10.1007/s00418-022-02081-5. Epub 2022 Mar 2. Histochem Cell Biol. 2022. PMID: 35235045 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

