GnRH Stimulates Peptidylarginine Deiminase Catalyzed Histone Citrullination in Gonadotrope Cells
- PMID: 27603413
- PMCID: PMC5045497
- DOI: 10.1210/me.2016-1085
GnRH Stimulates Peptidylarginine Deiminase Catalyzed Histone Citrullination in Gonadotrope Cells
Abstract
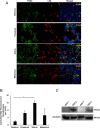
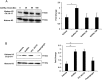
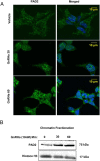
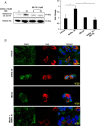
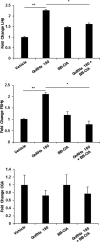
Peptidylarginine deiminase (PAD) enzymes convert histone tail arginine residues to citrulline resulting in chromatin decondensation. Our previous work found that PAD isoforms are expressed in female reproductive tissues in an estrous cycle-dependent fashion, but their role in the anterior pituitary gland is unknown. Thus, we investigated PAD expression and function in gonadotrope cells. The gonadotrope-derived LβT2 cell line strongly expresses PAD2 at the protein level compared with other PAD isoforms. Consistent with this, PAD2 protein expression is highest during the estrous phase of the estrous cycle and colocalizes with the LH β-subunit in the mouse pituitary. Using the GnRH agonist buserelin (GnRHa), studies in LβT2 and mouse primary gonadotrope cells revealed that 30 minutes of stimulation caused distinct puncta of PAD2 to localize in the nucleus. Once in the nucleus, GnRHa stimulated PAD2 citrullinates histone H3 tail arginine residues at sites 2, 8, and 17 within 30 minutes; however, this effect and PAD2 nuclear localization was blunted by incubation of the cells with the pan-PAD inhibitor, biphenyl-benzimidazole-Cl-amidine. Given that PAD2 citrullinates histones in gonadotropes, we next analyzed the functional consequence of PAD2 inhibition on gene expression. Our results show that GnRHa stimulates an increase in LHβ and FSHβ mRNA and that this response is significantly reduced in the presence of the PAD inhibitor biphenyl-benzimidazole-Cl-amidine. Overall, our data suggest that GnRHa stimulates PAD2-catalyzed histone citrullination in gonadotropes to epigenetically regulate gonadotropin gene expression.
Figures

Similar articles
-
GnRH Induces Citrullination of the Cytoskeleton in Murine Gonadotrope Cells.Int J Mol Sci. 2024 Mar 10;25(6):3181. doi: 10.3390/ijms25063181. Int J Mol Sci. 2024. PMID: 38542155 Free PMC article.
-
Potential role for peptidylarginine deiminase 2 (PAD2) in citrullination of canine mammary epithelial cell histones.PLoS One. 2010 Jul 26;5(7):e11768. doi: 10.1371/journal.pone.0011768. PLoS One. 2010. PMID: 20668670 Free PMC article.
-
Citrullination regulates the expression of insulin-like growth factor-binding protein 1 (IGFBP1) in ovine uterine luminal epithelial cells.Reproduction. 2017 Jan;153(1):1-10. doi: 10.1530/REP-16-0494. Epub 2016 Oct 10. Reproduction. 2017. PMID: 29565015 Free PMC article.
-
Peptidylarginine deiminase enzymes and citrullinated proteins in female reproductive physiology and associated diseases†.Biol Reprod. 2022 Dec 10;107(6):1395-1410. doi: 10.1093/biolre/ioac173. Biol Reprod. 2022. PMID: 36087287 Free PMC article. Review.
-
Possible role of PACAP and its PAC1 receptor in the differential regulation of pituitary LHbeta- and FSHbeta-subunit gene expression by pulsatile GnRH stimulation.Biol Reprod. 2013 Feb 14;88(2):35. doi: 10.1095/biolreprod.112.105601. Print 2013 Feb. Biol Reprod. 2013. PMID: 23197164 Review.
Cited by
-
The virtues and vices of protein citrullination.R Soc Open Sci. 2022 Jun 8;9(6):220125. doi: 10.1098/rsos.220125. eCollection 2022 Jun. R Soc Open Sci. 2022. PMID: 35706669 Free PMC article. Review.
-
Identification and Characterization of the Lactating Mouse Mammary Gland Citrullinome.Int J Mol Sci. 2020 Apr 10;21(7):2634. doi: 10.3390/ijms21072634. Int J Mol Sci. 2020. PMID: 32290104 Free PMC article.
-
Extracellular DNA Traps: Origin, Function and Implications for Anti-Cancer Therapies.Front Oncol. 2022 Apr 27;12:869706. doi: 10.3389/fonc.2022.869706. eCollection 2022. Front Oncol. 2022. PMID: 35574410 Free PMC article. Review.
-
GnRH Induces Citrullination of the Cytoskeleton in Murine Gonadotrope Cells.Int J Mol Sci. 2024 Mar 10;25(6):3181. doi: 10.3390/ijms25063181. Int J Mol Sci. 2024. PMID: 38542155 Free PMC article.
-
Histone Citrullination Represses MicroRNA Expression, Resulting in Increased Oncogene mRNAs in Somatolactotrope Cells.Mol Cell Biol. 2018 Sep 14;38(19):e00084-18. doi: 10.1128/MCB.00084-18. Print 2018 Oct 1. Mol Cell Biol. 2018. PMID: 29987187 Free PMC article.
References
-
- Naor Z. Signaling by G-protein-coupled receptor (GPCR): studies on the GnRH receptor. Front Neuroendocrinol. 2009;30:10–29. - PubMed
-
- Pernasetti F, Vasilyev VV, Rosenberg SB, et al. Cell-specific transcriptional regulation of FSHb by activin and GnRH in the LbT2 pituitary gonadotrope cell model. Endocrinology. 2001;142:2284–2295. - PubMed
-
- Ferris HA, Walsh HE, Stevens J, Fallest PC, Shupnik MA. Luteinizing hormone β promoter stimulation by adenylyl cyclase and cooperation with gonadotropin-releasing hormone 1 in transgenic mice and LβT2 Cells. Biol Reprod. 2007;77:1073–1080. - PubMed
-
- Mouillet JF, Sonnenberg-Hirche C, Yan X, Sadovsky Y. p300 regulates the synergy of steroidogenic factor-1 and early growth response-1 in activating luteinizing hormone-β subunit gene. J Biol Chem. 2004;279:7832–7839. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

