Human cytomegalovirus encoded chemokine receptor US28 activates the HIF-1α/PKM2 axis in glioblastoma cells
- PMID: 27602585
- PMCID: PMC5356532
- DOI: 10.18632/oncotarget.11817
Human cytomegalovirus encoded chemokine receptor US28 activates the HIF-1α/PKM2 axis in glioblastoma cells
Abstract
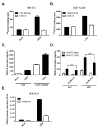
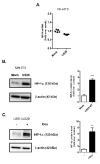
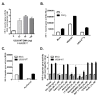
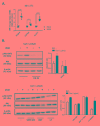
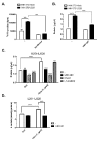
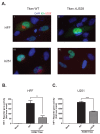
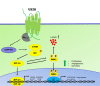
The human cytomegalovirus (HCMV) encoded chemokine receptor US28 promotes tumorigenesis through activation of various proliferative and angiogenic signaling pathways. Upon infection, US28 displays constitutive activity and signals in a G protein-dependent manner, hijacking the host's cellular machinery. In tumor cells, the hypoxia inducible factor-1α/pyruvate kinase M2 (HIF-1α/PKM2) axis plays an important role by supporting proliferation, angiogenesis and reprogramming of energy metabolism. In this study we show that US28 signaling results in activation of the HIF-1α/PKM2 feedforward loop in fibroblasts and glioblastoma cells. The constitutive activity of US28 increases HIF-1 protein stability through a Gαq-, CaMKII- and Akt/mTOR-dependent mechanism. Furthermore, we found that VEGF and lactate secretion are increased and HIF-1 target genes, glucose transporter type 1 (GLUT1) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), involved in glucose metabolism, are upregulated in US28 expressing cells. In addition, PKM2 is phosphorylated and found to be in a tumor-associated dimeric state upon US28 expression. Also in HCMV-infected cells HIF-1 activity is enhanced, which in part is US28-dependent. Finally, increased proliferation of cells expressing US28 is abolished upon inhibition of the HIF-1α/PKM2 cascade. These data highlight the importance of HIF-1α and PKM2 in US28-induced proliferation, angiogenesis and metabolic reprogramming.
Keywords: G protein-coupled receptor (GPCR); chemokine; glioblastoma; human cytomegalovirus (HCMV); hypoxia-inducible factor (HIF).
Conflict of interest statement
The authors declare no conflicts of interest in relation to this manuscript.
Figures

Similar articles
-
The human cytomegalovirus-encoded G protein-coupled receptor UL33 exhibits oncomodulatory properties.J Biol Chem. 2019 Nov 1;294(44):16297-16308. doi: 10.1074/jbc.RA119.007796. Epub 2019 Sep 13. J Biol Chem. 2019. PMID: 31519750 Free PMC article.
-
PHD3 is a transcriptional coactivator of HIF-1α in nucleus pulposus cells independent of the PKM2-JMJD5 axis.FASEB J. 2017 Sep;31(9):3831-3847. doi: 10.1096/fj.201601291R. Epub 2017 May 11. FASEB J. 2017. PMID: 28495754 Free PMC article.
-
Human cytomegalovirus-encoded chemokine receptor US28 promotes tumorigenesis.Proc Natl Acad Sci U S A. 2006 Aug 29;103(35):13068-73. doi: 10.1073/pnas.0604433103. Epub 2006 Aug 21. Proc Natl Acad Sci U S A. 2006. PMID: 16924106 Free PMC article.
-
PKM2 contributes to cancer metabolism.Cancer Lett. 2015 Jan 28;356(2 Pt A):184-91. doi: 10.1016/j.canlet.2014.01.031. Epub 2014 Feb 4. Cancer Lett. 2015. PMID: 24508027 Review.
-
Human Cytomegalovirus US28: a functionally selective chemokine binding receptor.Infect Disord Drug Targets. 2009 Nov;9(5):548-56. doi: 10.2174/187152609789105696. Infect Disord Drug Targets. 2009. PMID: 19594424 Free PMC article. Review.
Cited by
-
Selective targeting of ligand-dependent and -independent signaling by GPCR conformation-specific anti-US28 intrabodies.Nat Commun. 2021 Jul 16;12(1):4357. doi: 10.1038/s41467-021-24574-y. Nat Commun. 2021. PMID: 34272386 Free PMC article.
-
US28: HCMV's Swiss Army Knife.Viruses. 2018 Aug 20;10(8):445. doi: 10.3390/v10080445. Viruses. 2018. PMID: 30127279 Free PMC article. Review.
-
Accelerated cancer aggressiveness by viral oncomodulation: New targets and newer natural treatments for cancer control and treatment.Surg Neurol Int. 2019 Oct 11;10:199. doi: 10.25259/SNI_361_2019. eCollection 2019. Surg Neurol Int. 2019. PMID: 31768279 Free PMC article. Review.
-
Impact of Hypoxia over Human Viral Infections and Key Cellular Processes.Int J Mol Sci. 2021 Jul 26;22(15):7954. doi: 10.3390/ijms22157954. Int J Mol Sci. 2021. PMID: 34360716 Free PMC article. Review.
-
Clinical implications of cytomegalovirus in glioblastoma progression and therapy.NPJ Precis Oncol. 2024 Sep 29;8(1):213. doi: 10.1038/s41698-024-00709-4. NPJ Precis Oncol. 2024. PMID: 39343770 Free PMC article. Review.
References
-
- Gandhi MK, Khanna R. Human cytomegalovirus: clinical aspects, immune regulation, and emerging treatments. The Lancet Infectious diseases. 2004;4:725–738. - PubMed
-
- White MK, Gorrill TS, Khalili K. Reciprocal transactivation between HIV-1 and other human viruses. Virology. 2006;352:1–13. - PubMed
-
- Fischer SA. Emerging viruses in transplantation: there is more to infection after transplant than CMV and EBV. Transplantation. 2008;86:1327–1339. - PubMed
-
- Vischer HF, Siderius M, Leurs R, Smit MJ. Herpesvirus-encoded GPCRs: neglected players in inflammatory and proliferative diseases? Nat Rev Drug Discov. 2014;13:123–139. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

