Differential impact of minimal residual disease negativity according to the salvage status in patients with relapsed/refractory B-cell acute lymphoblastic leukemia
- PMID: 27602508
- PMCID: PMC5321541
- DOI: 10.1002/cncr.30264
Differential impact of minimal residual disease negativity according to the salvage status in patients with relapsed/refractory B-cell acute lymphoblastic leukemia
Abstract
Background: Minimal residual disease (MRD) assessment predicts survival for patients with newly diagnosed acute lymphoblastic leukemia (ALL). Its significance in relapsed/refractory ALL is less clear.
Methods: This study identified 78 patients with relapsed/refractory B-cell ALL who achieved a morphologic response with inotuzumab ozogamicin (n = 41), blinatumomab (n = 11), or mini-hyperfractionated cyclophosphamide, vincristine, and doxorubicin plus inotuzumab (n = 26) during either salvage 1 (S1; n = 46) or salvage 2 (S2; n = 32) and had undergone an MRD assessment by multiparameter flow cytometry at the time of remission.
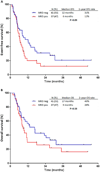
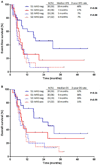
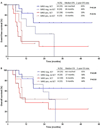
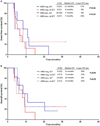
Results: MRD negativity was achieved in 41 patients overall (53%). The MRD negativity rate was 57% in S1 and 47% in S2. Among patients in S1, achieving MRD negativity was associated with longer event-free survival (EFS; median, 18 vs 7 months; 2-year EFS rate, 46% vs 17%; P = .06) and overall survival (OS; median, 27 vs 9 months; 2-year OS, 52% vs 36%; P = .15). EFS and OS were similar in S2, regardless of the MRD response. Among MRD-negative patients who underwent allogeneic stem cell transplantation (SCT), EFS and OS were superior for those who underwent SCT in S1 rather than S2 (P = .003 and P = .04, respectively). Patients in S1 who achieved MRD negativity and subsequently underwent SCT had the best outcomes with a 2-year OS rate of 65%.
Conclusions: Patients with relapsed/refractory ALL who achieve MRD negativity in S1 can have long-term survival. Patients in S2 generally have poor outcomes, regardless of their MRD status. Cancer 2017;123:294-302. © 2016 American Cancer Society.
Keywords: acute lymphoblastic leukemia; blinatumomab; inotuzumab; minimal residual disease; refractory; relapsed.
© 2016 American Cancer Society.
Figures

Similar articles
-
Impact of minimal residual disease status in patients with relapsed/refractory acute lymphoblastic leukemia treated with inotuzumab ozogamicin in the phase III INO-VATE trial.Leuk Res. 2020 Jan;88:106283. doi: 10.1016/j.leukres.2019.106283. Epub 2019 Nov 25. Leuk Res. 2020. PMID: 31790983 Clinical Trial.
-
Chemoimmunotherapy with inotuzumab ozogamicin combined with mini-hyper-CVD, with or without blinatumomab, is highly effective in patients with Philadelphia chromosome-negative acute lymphoblastic leukemia in first salvage.Cancer. 2018 Oct 15;124(20):4044-4055. doi: 10.1002/cncr.31720. Epub 2018 Oct 11. Cancer. 2018. PMID: 30307611 Free PMC article. Clinical Trial.
-
Salvage Chemoimmunotherapy With Inotuzumab Ozogamicin Combined With Mini-Hyper-CVD for Patients With Relapsed or Refractory Philadelphia Chromosome-Negative Acute Lymphoblastic Leukemia: A Phase 2 Clinical Trial.JAMA Oncol. 2018 Feb 1;4(2):230-234. doi: 10.1001/jamaoncol.2017.2380. JAMA Oncol. 2018. PMID: 28859185 Free PMC article. Clinical Trial.
-
Treatment with anti CD19 chimeric antigen receptor T cells after antibody-based immunotherapy in adults with acute lymphoblastic leukemia.Curr Res Transl Med. 2020 Jan;68(1):17-22. doi: 10.1016/j.retram.2019.12.001. Epub 2019 Dec 25. Curr Res Transl Med. 2020. PMID: 31882377 Review.
-
Taking a "BiTE out of ALL": blinatumomab approval for MRD-positive ALL.Blood. 2019 Apr 18;133(16):1715-1719. doi: 10.1182/blood-2018-12-852376. Epub 2019 Feb 22. Blood. 2019. PMID: 30796026 Free PMC article. Review.
Cited by
-
Consolidative Hematopoietic Stem Cell Transplantation After CD19 CAR-T Cell Therapy for Acute Lymphoblastic Leukemia: A Systematic Review and Meta-analysis.Front Oncol. 2021 Apr 28;11:651944. doi: 10.3389/fonc.2021.651944. eCollection 2021. Front Oncol. 2021. PMID: 34026627 Free PMC article.
-
Minimal Residual Disease in the Management of B-Cell Acute Lymphoblastic Leukemia: A Systematic Review of Studies from Indian Settings.Indian J Hematol Blood Transfus. 2024 Jan;40(1):1-11. doi: 10.1007/s12288-023-01641-6. Epub 2023 Mar 30. Indian J Hematol Blood Transfus. 2024. PMID: 38312181 Free PMC article. Review.
-
Superior survival outcome of blinatumomab compared with conventional chemotherapy for adult patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia: a propensity score-matched cohort analysis.Ther Adv Hematol. 2023 Mar 6;14:20406207231154713. doi: 10.1177/20406207231154713. eCollection 2023. Ther Adv Hematol. 2023. PMID: 36895914 Free PMC article.
-
[Current situation and prospect of minimal residual disease in acute myeloid leukemia].Zhonghua Xue Ye Xue Za Zhi. 2019 Jan 14;40(1):83-86. doi: 10.3760/cma.j.issn.0253-2727.2019.01.018. Zhonghua Xue Ye Xue Za Zhi. 2019. PMID: 30704237 Free PMC article. Chinese. No abstract available.
-
Novel monoclonal antibody-based treatment strategies in adults with acute lymphoblastic leukemia.Ther Adv Hematol. 2019 May 19;10:2040620719849496. doi: 10.1177/2040620719849496. eCollection 2019. Ther Adv Hematol. 2019. PMID: 31205644 Free PMC article. Review.
References
-
- Bruggemann M, Raff T, Flohr T, et al. Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. Blood. 2006;107(3):1116–1123. - PubMed
-
- Patel B, Rai L, Buck G, et al. Minimal residual disease is a significant predictor of treatment failure in non T-lineage adult acute lymphoblastic leukaemia: final results of the international trial UKALL XII/ECOG2993. Br J Haematol. 2010;148(1):80–89. - PubMed
-
- Ribera JM, Oriol A, Morgades M, et al. Treatment of high-risk Philadelphia chromosome-negative acute lymphoblastic leukemia in adolescents and adults according to early cytologic response and minimal residual disease after consolidation assessed by flow cytometry: final results of the PETHEMA ALL-AR-03 trial. J Clin Oncol. 2014;32(15):1595–1604. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

