Nicotine regulates activity of lateral habenula neurons via presynaptic and postsynaptic mechanisms
- PMID: 27596561
- PMCID: PMC5011770
- DOI: 10.1038/srep32937
Nicotine regulates activity of lateral habenula neurons via presynaptic and postsynaptic mechanisms
Abstract
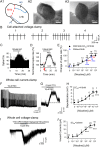
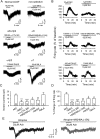
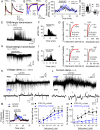
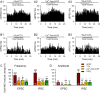
There is much interest in brain regions that drive nicotine intake in smokers. Interestingly, both the rewarding and aversive effects of nicotine are probably critical for sustaining nicotine addiction. The medial and lateral habenular (LHb) nuclei play important roles in processing aversion, and recent work has focused on the critical involvement of the LHb in encoding and responding to aversive stimuli. Several neurotransmitter systems are implicated in nicotine's actions, but very little is known about how nicotinic acetylcholine receptors (nAChRs) regulate LHb activity. Here we report in brain slices that activation of nAChRs depolarizes LHb cells and robustly increases firing, and also potentiates glutamate release in LHb. These effects were blocked by selective antagonists of α6-containing (α6*) nAChRs, and were absent in α6*-nAChR knockout mice. In addition, nicotine activates GABAergic inputs to LHb via α4β2-nAChRs, at lower concentrations but with more rapid desensitization relative to α6*-nAChRs. These results demonstrate the existence of diverse functional nAChR subtypes at presynaptic and postsynaptic sites in LHb, through which nicotine could facilitate or inhibit LHb neuronal activity and thus contribute to nicotine aversion or reward.
Figures

Similar articles
-
Neurobiological Mechanisms of Nicotine Reward and Aversion.Pharmacol Rev. 2022 Jan;74(1):271-310. doi: 10.1124/pharmrev.121.000299. Pharmacol Rev. 2022. PMID: 35017179 Free PMC article. Review.
-
Nicotinic activation of laterodorsal tegmental neurons: implications for addiction to nicotine.Neuropsychopharmacology. 2009 Nov;34(12):2529-47. doi: 10.1038/npp.2009.82. Epub 2009 Jul 22. Neuropsychopharmacology. 2009. PMID: 19625996 Free PMC article.
-
Habenula cholinergic neurons regulate anxiety during nicotine withdrawal via nicotinic acetylcholine receptors.Neuropharmacology. 2016 Aug;107:294-304. doi: 10.1016/j.neuropharm.2016.03.039. Epub 2016 Mar 26. Neuropharmacology. 2016. PMID: 27020042 Free PMC article.
-
Differential roles of α6β2* and α4β2* neuronal nicotinic receptors in nicotine- and cocaine-conditioned reward in mice.Neuropsychopharmacology. 2015 Jan;40(2):350-60. doi: 10.1038/npp.2014.177. Epub 2014 Jul 18. Neuropsychopharmacology. 2015. PMID: 25035086 Free PMC article.
-
Cellular mechanisms of nicotine addiction.Pharmacol Biochem Behav. 2001 Dec;70(4):439-46. doi: 10.1016/s0091-3057(01)00652-9. Pharmacol Biochem Behav. 2001. PMID: 11796143 Review.
Cited by
-
Neurobiological Mechanisms of Nicotine Reward and Aversion.Pharmacol Rev. 2022 Jan;74(1):271-310. doi: 10.1124/pharmrev.121.000299. Pharmacol Rev. 2022. PMID: 35017179 Free PMC article. Review.
-
Acetaldehyde Excitation of Lateral Habenular Neurons via Multiple Cellular Mechanisms.J Neurosci. 2021 Sep 8;41(36):7532-7545. doi: 10.1523/JNEUROSCI.2913-20.2021. Epub 2021 Jul 29. J Neurosci. 2021. PMID: 34326141 Free PMC article.
-
From controlled to compulsive drug-taking: The role of the habenula in addiction.Neurosci Biobehav Rev. 2019 Nov;106:102-111. doi: 10.1016/j.neubiorev.2018.06.018. Epub 2018 Jun 21. Neurosci Biobehav Rev. 2019. PMID: 29936111 Free PMC article. Review.
-
The interaction of the Chrna5 D398N variant with developmental nicotine exposure.Genes Brain Behav. 2018 Sep;17(7):e12474. doi: 10.1111/gbb.12474. Epub 2018 Apr 17. Genes Brain Behav. 2018. PMID: 29573323 Free PMC article.
-
Activation of glycine receptors in the lateral habenula rescues anxiety- and depression-like behaviors associated with alcohol withdrawal and reduces alcohol intake in rats.Neuropharmacology. 2019 Oct;157:107688. doi: 10.1016/j.neuropharm.2019.107688. Epub 2019 Jun 27. Neuropharmacology. 2019. PMID: 31254534 Free PMC article.
References
-
- Kenny P. J. & Markou A. Neurobiology of the nicotine withdrawal syndrome. Pharmacol Biochem Behav 70, 531–549 (2001). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

