The anti-inflammatory Annexin A1 induces the clearance and degradation of the amyloid-β peptide
- PMID: 27590054
- PMCID: PMC5010757
- DOI: 10.1186/s12974-016-0692-6
The anti-inflammatory Annexin A1 induces the clearance and degradation of the amyloid-β peptide
Abstract
Background: The toxicity of amyloid-β (Aβ) peptide present in the brain of Alzheimer's disease (AD) patients is thought to be mediated via the increased secretion of pro-inflammatory mediators, which can lead to neuronal dysfunction and cell death. In addition, we have previously shown that inflammation can affect Aβ generation. More recently, we have reported that in vitro administration of the anti-inflammatory mediator Annexin A1 (ANXA1) following an inflammatory challenge suppressed microglial activation and this effect was mediated through formyl peptide receptor-like 1 (FPRL1/FPR2) signalling. The aim of this study was to determine the potential role of ANXA1 in the generation and clearance of Aβ.
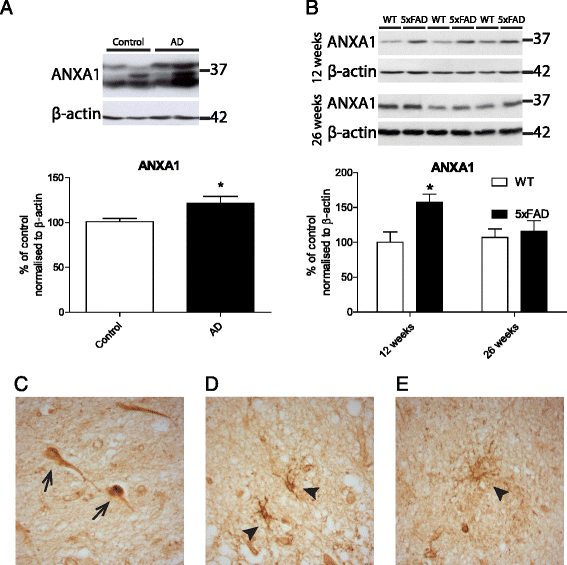
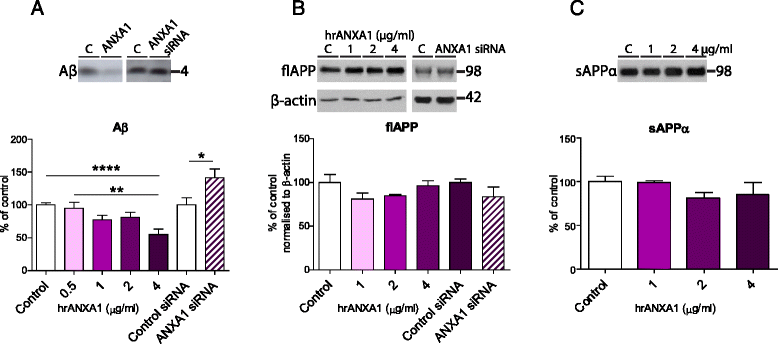
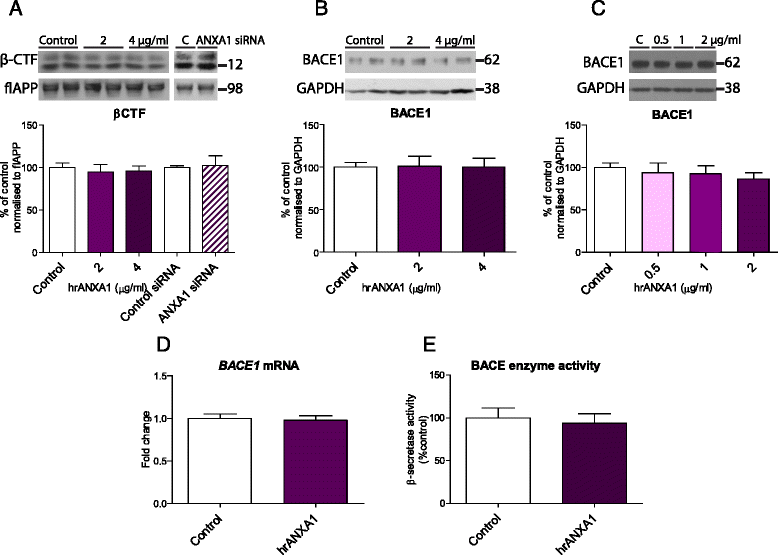
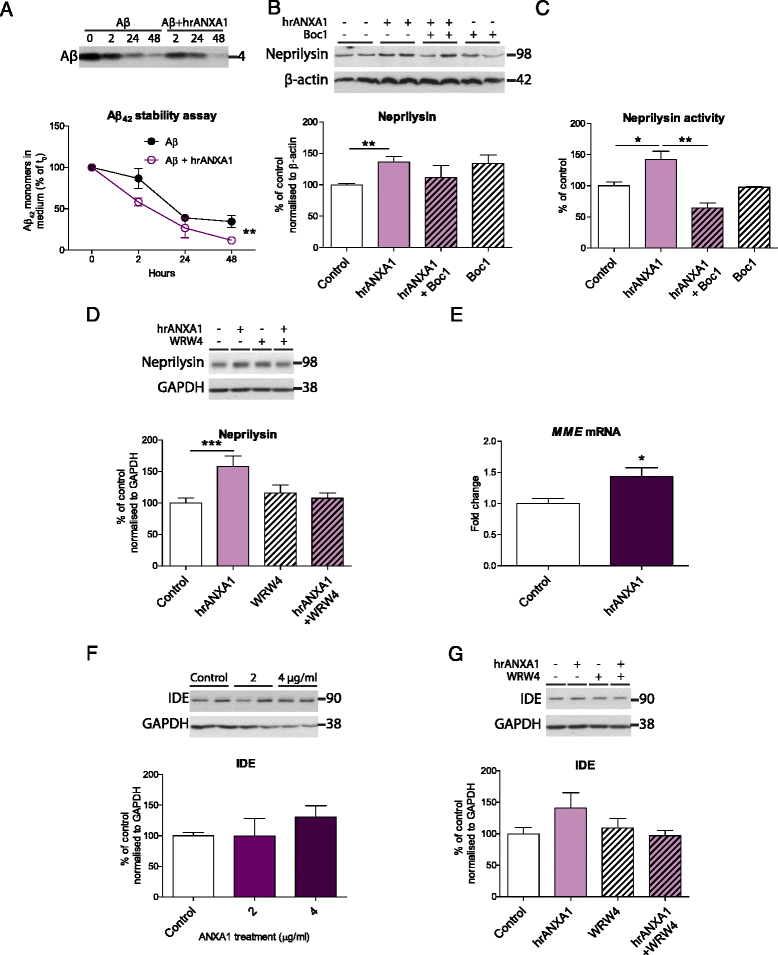
Methods: We first compared ANXA1 protein expression in the brains of AD patients and healthy controls as well as in the 5XFAD model of AD. To determine the role of ANXA1 in the processing of amyloid precursor protein (APP) and the degradation of Aβ, N2a neuroblastoma cells were treated with human recombinant ANXA1 or transfected with ANXA1 siRNA. We also investigated the effect of ANXA1 on Aβ phagocytosis and microglial activation in BV2 cells treated with synthetic Aβ.
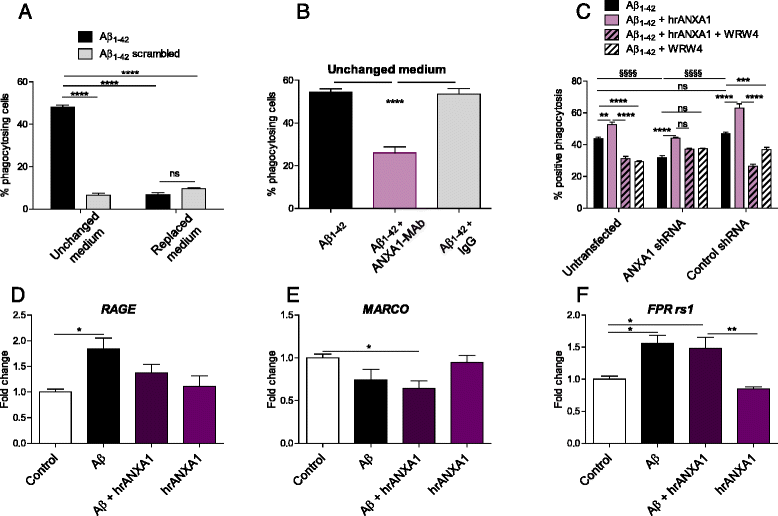
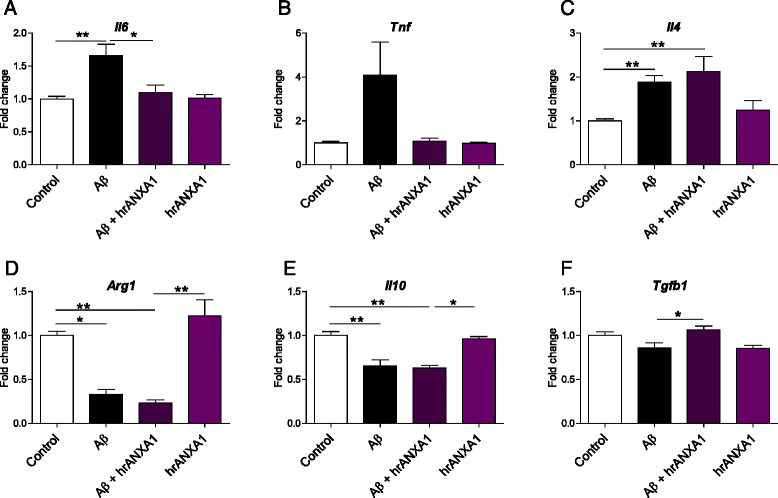
Results: Our data show that ANXA1 is increased in the brains of AD patients and animal models of AD at early stages. ANXA1 was able to reduce the levels of Aβ by increasing its enzymatic degradation by neprilysin in N2a cells and to stimulate Aβ phagocytosis by microglia. These effects were mediated through FPRL1 receptors. In addition, ANXA1 inhibited the Aβ-stimulated secretion of inflammatory mediators by microglia.
Conclusions: These data suggest that ANXA1 plays a pivotal role in Aβ clearance and supports the use of ANXA1 as potential pharmacological tool for AD therapeutics.
Keywords: Alzheimer’s disease; Amyloid-β; Annexin A1; Anti-inflammatory; Formyl-peptide receptor; Inflammation; Microglia; Neprilysin.
Figures
Similar articles
-
Annexin A1 restores Aβ1-42 -induced blood-brain barrier disruption through the inhibition of RhoA-ROCK signaling pathway.Aging Cell. 2017 Feb;16(1):149-161. doi: 10.1111/acel.12530. Epub 2016 Sep 16. Aging Cell. 2017. PMID: 27633771 Free PMC article.
-
Anti-inflammatory mechanisms of the annexin A1 protein and its mimetic peptide Ac2-26 in models of ocular inflammation in vivo and in vitro.J Immunol. 2013 Jun 1;190(11):5689-701. doi: 10.4049/jimmunol.1202030. Epub 2013 May 3. J Immunol. 2013. PMID: 23645879
-
Annexin A1 restores cerebrovascular integrity concomitant with reduced amyloid-β and tau pathology.Brain. 2021 Jun 22;144(5):1526-1541. doi: 10.1093/brain/awab050. Brain. 2021. PMID: 34148071 Free PMC article.
-
Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
Cited by
-
Connecting genomic and proteomic signatures of amyloid burden in the brain.medRxiv [Preprint]. 2024 Sep 6:2024.09.06.24313124. doi: 10.1101/2024.09.06.24313124. medRxiv. 2024. PMID: 39281766 Free PMC article. Preprint.
-
Importance of GPCR-Mediated Microglial Activation in Alzheimer's Disease.Front Cell Neurosci. 2018 Aug 21;12:258. doi: 10.3389/fncel.2018.00258. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30186116 Free PMC article. Review.
-
Annexin A1-derived peptide Ac2-26 in a pilocarpine-induced status epilepticus model: anti-inflammatory and neuroprotective effects.J Neuroinflammation. 2019 Feb 12;16(1):32. doi: 10.1186/s12974-019-1414-7. J Neuroinflammation. 2019. PMID: 30755225 Free PMC article.
-
The presubiculum is preserved from neurodegenerative changes in Alzheimer's disease.Acta Neuropathol Commun. 2018 Jul 20;6(1):62. doi: 10.1186/s40478-018-0563-8. Acta Neuropathol Commun. 2018. PMID: 30029687 Free PMC article.
-
The formyl peptide receptor agonist FPRa14 induces differentiation of Neuro2a mouse neuroblastoma cells into multiple distinct morphologies which can be specifically inhibited with FPR antagonists and FPR knockdown using siRNA.PLoS One. 2019 Jun 6;14(6):e0217815. doi: 10.1371/journal.pone.0217815. eCollection 2019. PLoS One. 2019. PMID: 31170199 Free PMC article.
References
-
- Sastre M, Dewachter I, Landreth GE, Willson TM, Klockgether T, van Leuven F, et al. Nonsteroidal anti-inflammatory drugs and peroxisome proliferator-activated receptor-gamma agonists modulate immunostimulated processing of amyloid precursor protein through regulation of beta-secretase. J Neurosci. 2003;23:9796–9804. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

