Antitumor Activity of Cytotoxic Cyclooxygenase-2 Inhibitors
- PMID: 27588346
- PMCID: PMC5289892
- DOI: 10.1021/acschembio.6b00560
Antitumor Activity of Cytotoxic Cyclooxygenase-2 Inhibitors
Abstract
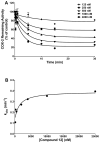
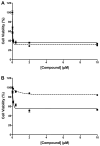
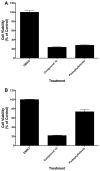
Targeted delivery of chemotherapeutic agents to tumors has been explored as a means to increase the selectivity and potency of cytotoxicity. Most efforts in this area have exploited the molecular recognition of proteins highly expressed on the surface of cancer cells followed by internalization. A related approach that has received less attention is the targeting of intracellular proteins by ligands conjugated to anticancer drugs. An attractive target for this approach is the enzyme cyclooxygenase-2 (COX-2), which is highly expressed in a range of malignant tumors. Herein, we describe the synthesis and evaluation of a series of chemotherapeutic agents targeted to COX-2 by conjugation to indomethacin. Detailed characterization of compound 12, a conjugate of indomethacin with podophyllotoxin, revealed highly potent and selective COX-2 inhibition in vitro and in intact cells. Kinetics and X-ray crystallographic studies demonstrated that compound 12 is a slow, tight-binding inhibitor that likely binds to COX-2's allosteric site with its indomethacin moiety in a conformation similar to that of indomethacin. Compound 12 exhibited cytotoxicity in cell culture similar to that of podophyllotoxin with no evidence of COX-2-dependent selectivity. However, in vivo, compound 12 accumulated selectively in and more effectively inhibited the growth of a COX-2-expressing xenograft compared to a xenograft that did not express COX-2. Compound 12, which we have named chemocoxib A, provides proof-of-concept for the in vivo targeting of chemotherapeutic agents to COX-2 but suggests that COX-2-dependent selectivity may not be evident in cell culture-based assays.
Figures
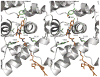
Similar articles
-
Cyclooxygenase inhibitors decrease the growth and induce regression of human esophageal adenocarcinoma xenografts in nude mice.Int J Oncol. 2012 Feb;40(2):527-34. doi: 10.3892/ijo.2011.1219. Epub 2011 Oct 3. Int J Oncol. 2012. PMID: 21971589
-
Fluorescent indomethacin-dansyl conjugates utilize the membrane-binding domain of cyclooxygenase-2 to block the opening to the active site.J Biol Chem. 2019 May 31;294(22):8690-8698. doi: 10.1074/jbc.RA119.007405. Epub 2019 Apr 18. J Biol Chem. 2019. PMID: 31000626 Free PMC article.
-
Biochemical and pharmacological profile of a tetrasubstituted furanone as a highly selective COX-2 inhibitor.Br J Pharmacol. 1997 May;121(1):105-17. doi: 10.1038/sj.bjp.0701076. Br J Pharmacol. 1997. PMID: 9146894 Free PMC article.
-
Design and synthesis of benzimidazole analogs endowed with oxadiazole as selective COX-2 inhibitor.Arch Pharm (Weinheim). 2014 Dec;347(12):923-35. doi: 10.1002/ardp.201400219. Epub 2014 Oct 9. Arch Pharm (Weinheim). 2014. PMID: 25303727
-
Targeted cancer therapy: conferring specificity to cytotoxic drugs.Acc Chem Res. 2008 Jan;41(1):98-107. doi: 10.1021/ar700108g. Epub 2007 Aug 18. Acc Chem Res. 2008. PMID: 17705444 Review.
Cited by
-
Strategy to targeting the immune resistance and novel therapy in colorectal cancer.Cancer Med. 2018 May;7(5):1578-1603. doi: 10.1002/cam4.1386. Epub 2018 Apr 15. Cancer Med. 2018. PMID: 29658188 Free PMC article. Review.
-
Structural and Chemical Biology of the Interaction of Cyclooxygenase with Substrates and Non-Steroidal Anti-Inflammatory Drugs.Chem Rev. 2020 Aug 12;120(15):7592-7641. doi: 10.1021/acs.chemrev.0c00215. Epub 2020 Jul 1. Chem Rev. 2020. PMID: 32609495 Free PMC article. Review.
-
Targeted Detection of Cyclooxygenase-1 in Ovarian Cancer.ACS Med Chem Lett. 2019 Jul 24;11(10):1837-1842. doi: 10.1021/acsmedchemlett.9b00280. eCollection 2020 Oct 8. ACS Med Chem Lett. 2019. PMID: 33062161 Free PMC article.
-
Evaluation of Selective COX-2 Inhibition and In Silico Study of Kuwanon Derivatives Isolated from Morus alba.Int J Mol Sci. 2021 Apr 1;22(7):3659. doi: 10.3390/ijms22073659. Int J Mol Sci. 2021. PMID: 33915826 Free PMC article.
-
In vivo determination of analgesic and anti-inflammatory activities of isolated compounds from Cleome amblyocarpa and molecular modelling for the top active investigated compounds.RSC Adv. 2024 Aug 5;14(34):24503-24515. doi: 10.1039/d4ra04496g. eCollection 2024 Aug 5. RSC Adv. 2024. PMID: 39108954 Free PMC article.
References
-
- Vlahov IR, Leamon CP. Engineering folate-drug conjugates to target cancer: from chemistry to clinic. Bioconjug Chem. 2012;23:1357–1369. - PubMed
-
- Abdalla SI, Lao-Sirieix P, Novelli MR, Lovat LB, Sanderson IR, Fitzgerald RC. Gastrin-induced cyclooxygenase-2 expression in Barrett’s carcinogenesis. Clin Cancer Res. 2004;10:4784–4792. - PubMed
-
- Sano H, Kawahito Y, Wilder RL, Hashiramoto A, Mukai S, Asai K, Kimura S, Kato H, Kondo M, Hla T. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res. 1995;55:3785–3789. - PubMed
-
- Shirvani VN, Ouatu-Lascar R, Kaur BS, Omary MB, Triadafilopoulos G. Cyclooxygenase 2 expression in Barrett’s esophagus and adenocarcinoma: Ex vivo induction by bile salts and acid exposure. Gastroenterology. 2000;118:487–496. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Research Materials

