Evaluation of expression of the Wnt signaling components in canine mammary tumors via RT2 Profiler PCR Array and immunochemistry assays
- PMID: 27586466
- PMCID: PMC5639089
- DOI: 10.4142/jvs.2017.18.3.359
Evaluation of expression of the Wnt signaling components in canine mammary tumors via RT2 Profiler PCR Array and immunochemistry assays
Abstract
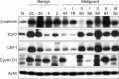
The Wnt signaling pathway and its key component β-catenin have critical roles in the development of diseases such as tumors in mammals. However, little has been reported about involvement of the Wnt/β-catenin signaling pathway in canine mammary tumors (CMTs). The present study detected expression of 30 Wnt signaling pathway-related genes in CMTs; the results are potentially useful for molecular-based diagnosis of CMTs and the development of new targeted therapies. Significant upregulations of dickkopf-1 protein, secreted frizzled-related sequence protein 1 (SFRP1), frizzled 3, β-catenin, and lymphoid enhancer-binding factor 1 (LEF1) were detected in highly malignant CMTs compared to levels in normal mammary gland tissues; moreover, highly significant upregulation of WNT5A was observed in low malignancy CMTs. Downregulation was only detected for SFRP4 in malignant CMT samples. The subcellular location of β-catenin and cyclin D1 in 100 CMT samples was investigated via immunohistochemical analysis, and significantly increased expressions of β-catenin in cytoplasm and cyclin D1 in nuclei were revealed. Western blotting analysis revealed that the expression of β-catenin and LEF1 increased in in the majority of CMT samples. Taken together, the results provide important evidence of the activation status of the Wnt pathway in CMTs and valuable clues to identifying biomarkers for molecular-based diagnosis of CMT.
Keywords: RT2 Profiler PCR Array; Wnt signaling pathway; beta-catenin; canine mammary tumor.
Conflict of interest statement
Figures
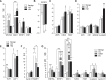


Similar articles
-
Secreted frizzled-related protein 2 (SFRP2) is highly expressed in canine mammary gland tumors but not in normal mammary glands.Breast Cancer Res Treat. 2004 Mar;84(2):139-49. doi: 10.1023/B:BREA.0000018412.83348.ff. Breast Cancer Res Treat. 2004. PMID: 14999144
-
Survivin and related proteins in canine mammary tumors: immunohistochemical expression.Vet Pathol. 2015 Mar;52(2):269-75. doi: 10.1177/0300985814529312. Epub 2014 Mar 31. Vet Pathol. 2015. PMID: 24686389
-
Expression of secreted frizzled-related protein 2 in a primary canine mammary tumor cell line: a candidate tumor marker for mammary tumor cells.In Vitro Cell Dev Biol Anim. 2003 May-Jun;39(5-6):221-7. doi: 10.1290/1543-706X(2003)039<0221:EOSFPI>2.0.CO;2. In Vitro Cell Dev Biol Anim. 2003. PMID: 12866949
-
Distinctive microRNA signature associated of neoplasms with the Wnt/β-catenin signaling pathway.Cell Signal. 2013 Dec;25(12):2805-11. doi: 10.1016/j.cellsig.2013.09.006. Epub 2013 Sep 13. Cell Signal. 2013. PMID: 24041653 Review.
-
Exploring the role of microRNAs as diagnostic and prognostic biomarkers in canine mammary tumors.Geroscience. 2024 Dec;46(6):6641-6657. doi: 10.1007/s11357-024-01260-7. Epub 2024 Jul 2. Geroscience. 2024. PMID: 38954129 Free PMC article. Review.
Cited by
-
The Expression of Selected Wnt Pathway Members (FZD6, AXIN2 and β-Catenin) in Canine Oral Squamous Cell Carcinoma and Acanthomatous Ameloblastoma.Animals (Basel). 2021 May 29;11(6):1615. doi: 10.3390/ani11061615. Animals (Basel). 2021. PMID: 34072517 Free PMC article.
-
Immunophenotypic Characterization of Canine Splenic Follicular-Derived B-Cell Lymphoma.Vet Pathol. 2019 May;56(3):350-357. doi: 10.1177/0300985818823668. Epub 2019 Jan 13. Vet Pathol. 2019. PMID: 30636524 Free PMC article.
-
SFRP1 Expression is Inversely Associated With Metastasis Formation in Canine Mammary Tumours.J Mammary Gland Biol Neoplasia. 2023 Jul 4;28(1):15. doi: 10.1007/s10911-023-09543-z. J Mammary Gland Biol Neoplasia. 2023. PMID: 37402051 Free PMC article.
-
Antitumor Effect of Berberine Analogs in a Canine Mammary Tumor Cell Line and in Zebrafish Reporters via Wnt/β-Catenin and Hippo Pathways.Biomedicines. 2023 Dec 15;11(12):3317. doi: 10.3390/biomedicines11123317. Biomedicines. 2023. PMID: 38137538 Free PMC article.
-
Notch Signaling Pathway Expression in the Skin of Leprosy Patients: Association With Skin and Neural Damage.Front Immunol. 2020 Mar 19;11:368. doi: 10.3389/fimmu.2020.00368. eCollection 2020. Front Immunol. 2020. PMID: 32265900 Free PMC article.
References
-
- Brunetti B, Sarli G, Preziosi R, Monari I, Benazzi C. E-cadherin and β-catenin reduction influence invasion but not proliferation and survival in canine malignant mammary tumors. Vet Pathol. 2005;42:781–787. - PubMed
-
- Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127:469–480. - PubMed
-
- Cuello-Carrión FD, Shortrede JE, Alvarez-Olmedo D, Cayado-Gutiérrez N, Castro GN, Zoppino FCM, Guerrero M, Martinis E, Wuilloud R, Gómez NN, Biaggio V, Orozco J, Gago FE, Ciocca LA, Fanelli MA, Ciocca DR. HER2 and β-catenin protein location: importance in the prognosis of breast cancer patients and their correlation when breast cancer cells suffer stressful situations. Clin Exp Metastasis. 2015;32:151–168. - PubMed
-
- Gama A, Paredes J, Gärtner F, Alves A, Schmitt F. Expression of E-cadherin, P-cadherin and β-catenin in canine malignant mammary tumours in relation to clinicopathological parameters, proliferation and survival. Vet J. 2008;177:45–53. - PubMed
-
- Goldschmidt M, Peña L, Rasotto R, Zappulli V. Classification and grading of canine mammary tumors. Vet Pathol. 2011;48:117–131. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

