Resolving Isomeric Glycopeptide Glycoforms with Hydrophilic Interaction Chromatography (HILIC)
- PMID: 27582638
- PMCID: PMC4972402
- DOI: 10.7171/jbt.16-2703-003
Resolving Isomeric Glycopeptide Glycoforms with Hydrophilic Interaction Chromatography (HILIC)
Abstract
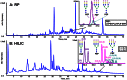
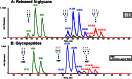
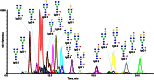
The ability to resolve glycans while attached to tryptic peptides would greatly facilitate glycoproteomics, as this would enable site-specific glycan characterization. Peptide/glycopeptide separations are typically performed using reversed-phase liquid chromatography (RPLC), where retention is driven by hydrophobic interaction. As the hydrophilic glycans do not interact significantly with the RPLC stationary phase, it is difficult to resolve glycopeptides that differ only in their glycan structure, even when these differences are large. Alternatively, glycans interact extensively with the stationary phases used in hydrophilic interaction chromatography (HILIC), and consequently, differences in glycan structure have profound chromatographic shifts in this chromatographic mode. Here, we evaluate HILIC for the separation of isomeric glycopeptide mixtures that have the same peptide backbone but isomeric glycans. Hydrophilic functional groups on both the peptide and the glycan interact with the HILIC stationary phase, and thus, changes to either of these moieties can alter the chromatographic behavior of a glycopeptide. The interactive processes permit glycopeptides to be resolved from each other based on differences in their amino acid sequences and/or their attached glycans. The separations of glycans in HILIC are sufficient to permit resolution of isomeric N-glycan structures, such as sialylated N-glycan isomers differing in α2-3 and α2-6 linkages, while these glycans remain attached to peptides.
Keywords: Fetuin; HILIC; IgGs; Isomeric Separation.
Figures

Similar articles
-
Comparison of Different HILIC Stationary Phases in the Separation of Hemopexin and Immunoglobulin G Glycopeptides and Their Isomers.Molecules. 2020 Oct 13;25(20):4655. doi: 10.3390/molecules25204655. Molecules. 2020. PMID: 33065988 Free PMC article.
-
Comparison of human IgG glycopeptides separation using mixed-mode hydrophilic interaction/ion-exchange liquid chromatography and reversed-phase mode.Anal Bioanal Chem. 2021 Jul;413(16):4321-4328. doi: 10.1007/s00216-021-03388-3. Epub 2021 May 17. Anal Bioanal Chem. 2021. PMID: 34002272
-
A review of intact glycopeptide enrichment and glycan separation through hydrophilic interaction liquid chromatography stationary phase materials.J Chromatogr A. 2024 Oct 25;1735:465318. doi: 10.1016/j.chroma.2024.465318. Epub 2024 Aug 31. J Chromatogr A. 2024. PMID: 39244913 Review.
-
Separation of isomeric 2-aminopyridine derivatized N-glycans and N-glycopeptides of human serum immunoglobulin G by using a zwitterionic type of hydrophilic-interaction chromatography.J Chromatogr A. 2006 Apr 28;1113(1-2):177-81. doi: 10.1016/j.chroma.2006.02.010. Epub 2006 Feb 28. J Chromatogr A. 2006. PMID: 16503336
-
Glycomics and glycoproteomics: Approaches to address isomeric separation of glycans and glycopeptides.J Sep Sci. 2021 Jan;44(1):403-425. doi: 10.1002/jssc.202000878. Epub 2020 Dec 14. J Sep Sci. 2021. PMID: 33090644 Review.
Cited by
-
Early Stage Glycosylation Biomarkers in Alzheimer's Disease.Medicines (Basel). 2019 Sep 3;6(3):92. doi: 10.3390/medicines6030092. Medicines (Basel). 2019. PMID: 31484367 Free PMC article. Review.
-
In-depth structural analysis of glycans in the gas phase.Chem Sci. 2019 Jan 4;10(5):1272-1284. doi: 10.1039/c8sc05426f. eCollection 2019 Feb 7. Chem Sci. 2019. PMID: 30809341 Free PMC article. Review.
-
Comparison of Different HILIC Stationary Phases in the Separation of Hemopexin and Immunoglobulin G Glycopeptides and Their Isomers.Molecules. 2020 Oct 13;25(20):4655. doi: 10.3390/molecules25204655. Molecules. 2020. PMID: 33065988 Free PMC article.
-
Mass Spectrometry Approaches to Glycomic and Glycoproteomic Analyses.Chem Rev. 2018 Sep 12;118(17):7886-7930. doi: 10.1021/acs.chemrev.7b00732. Epub 2018 Mar 19. Chem Rev. 2018. PMID: 29553244 Free PMC article. Review.
-
Towards structure-focused glycoproteomics.Biochem Soc Trans. 2021 Feb 26;49(1):161-186. doi: 10.1042/BST20200222. Biochem Soc Trans. 2021. PMID: 33439247 Free PMC article. Review.
References
-
- Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell 2006;126:855–867. - PubMed
-
- Ray K, Clapp P, Goldsmith PK, Spiegel AM. Identification of the sites of N-linked glycosylation on the human calcium receptor and assessment of their role in cell surface expression and signal transduction. J Biol Chem 1998;273:34558–34567. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
