Identification of IGFBP2 and IGFBP3 As Compensatory Biomarkers for CA19-9 in Early-Stage Pancreatic Cancer Using a Combination of Antibody-Based and LC-MS/MS-Based Proteomics
- PMID: 27579675
- PMCID: PMC5007017
- DOI: 10.1371/journal.pone.0161009
Identification of IGFBP2 and IGFBP3 As Compensatory Biomarkers for CA19-9 in Early-Stage Pancreatic Cancer Using a Combination of Antibody-Based and LC-MS/MS-Based Proteomics
Abstract
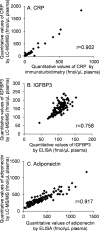
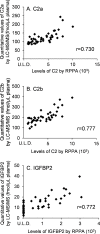
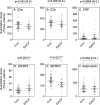
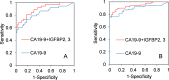
Pancreatic cancer is one of the most lethal tumors, and reliable detection of early-stage pancreatic cancer and risk diseases for pancreatic cancer is essential to improve the prognosis. As 260 genes were previously reported to be upregulated in invasive ductal adenocarcinoma of pancreas (IDACP) cells, quantification of the corresponding proteins in plasma might be useful for IDACP diagnosis. Therefore, the purpose of the present study was to identify plasma biomarkers for early detection of IDACP by using two proteomics strategies: antibody-based proteomics and liquid chromatography-tandem mass spectrometry (LC-MS/MS)-based proteomics. Among the 260 genes, we focused on 130 encoded proteins with known function for which antibodies were available. Twenty-three proteins showed values of the area under the curve (AUC) of more than 0.8 in receiver operating characteristic (ROC) analysis of reverse-phase protein array (RPPA) data of IDACP patients compared with healthy controls, and these proteins were selected as biomarker candidates. We then used our high-throughput selected reaction monitoring or multiple reaction monitoring (SRM/MRM) methodology, together with an automated sample preparation system, micro LC and auto analysis system, to quantify these candidate proteins in plasma from healthy controls and IDACP patients on a large scale. The results revealed that insulin-like growth factor-binding protein (IGFBP)2 and IGFBP3 have the ability to discriminate IDACP patients at an early stage from healthy controls, and IGFBP2 appeared to be increased in risk diseases of pancreatic malignancy, such as intraductal papillary mucinous neoplasms (IPMNs). Furthermore, diagnosis of IDACP using the combination of carbohydrate antigen 19-9 (CA19-9), IGFBP2 and IGFBP3 is significantly more effective than CA19-9 alone. This suggests that IGFBP2 and IGFBP3 may serve as compensatory biomarkers for CA19-9. Early diagnosis with this marker combination may improve the prognosis of IDACP patients.
Conflict of interest statement
The authors declare no competing financial interest. One of our authors, W.H., is a CEO of Abnova and receives salary from Abnova. He also owns stock in Abnova. This does not alter the authors’ adherence to PLOS ONE policies on sharing data and materials.
Figures

Similar articles
-
Sequential Validation of Blood-Based Protein Biomarker Candidates for Early-Stage Pancreatic Cancer.J Natl Cancer Inst. 2017 Apr 1;109(4):djw266. doi: 10.1093/jnci/djw266. J Natl Cancer Inst. 2017. PMID: 28376157 Free PMC article.
-
Validation of four candidate pancreatic cancer serological biomarkers that improve the performance of CA19.9.BMC Cancer. 2013 Sep 3;13:404. doi: 10.1186/1471-2407-13-404. BMC Cancer. 2013. PMID: 24007603 Free PMC article.
-
Development of a Fit-For-Purpose Multi-Marker Panel for Early Diagnosis of Pancreatic Ductal Adenocarcinoma.Mol Cell Proteomics. 2024 Sep;23(9):100824. doi: 10.1016/j.mcpro.2024.100824. Epub 2024 Aug 5. Mol Cell Proteomics. 2024. PMID: 39097268 Free PMC article.
-
Updates and Critical Evaluation on Novel Biomarkers for the Malignant Progression of Intraductal Papillary Mucinous Neoplasms of the Pancreas.Anticancer Res. 2017 May;37(5):2185-2194. doi: 10.21873/anticanres.11553. Anticancer Res. 2017. PMID: 28476781 Review.
-
Potential biomarkers for early detection of pancreatic ductal adenocarcinoma.Clin Transl Oncol. 2020 Dec;22(12):2170-2174. doi: 10.1007/s12094-020-02372-0. Epub 2020 May 23. Clin Transl Oncol. 2020. PMID: 32447642 Free PMC article. Review.
Cited by
-
Diagnostic accuracy and added value of blood-based protein biomarkers for pancreatic cancer: A meta-analysis of aggregate and individual participant data.EClinicalMedicine. 2022 Nov 24;55:101747. doi: 10.1016/j.eclinm.2022.101747. eCollection 2023 Jan. EClinicalMedicine. 2022. PMID: 36457649 Free PMC article.
-
Advances in pancreatic cancer biomarkers.Oncol Rev. 2019 Feb 1;13(1):410. doi: 10.4081/oncol.2019.410. eCollection 2019 Jan 14. Oncol Rev. 2019. PMID: 31044028 Free PMC article.
-
Validation of Biomarkers for Early Detection of Pancreatic Cancer: Summary of The Alliance of Pancreatic Cancer Consortia for Biomarkers for Early Detection Workshop.Pancreas. 2018 Feb;47(2):135-141. doi: 10.1097/MPA.0000000000000973. Pancreas. 2018. PMID: 29346214 Free PMC article.
-
Recent developments in mass-spectrometry-based targeted proteomics of clinical cancer biomarkers.Clin Proteomics. 2024 Jan 30;21(1):6. doi: 10.1186/s12014-024-09452-1. Clin Proteomics. 2024. PMID: 38287260 Free PMC article. Review.
-
Familial Pancreatic Cancer: Current Perspectives.Cancer Manag Res. 2020 Jan 31;12:743-758. doi: 10.2147/CMAR.S172421. eCollection 2020. Cancer Manag Res. 2020. PMID: 32099470 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

