TRF2 is recruited to the pre-initiation complex as a testis-specific subunit of TFIIA/ALF to promote haploid cell gene expression
- PMID: 27576952
- PMCID: PMC5006001
- DOI: 10.1038/srep32069
TRF2 is recruited to the pre-initiation complex as a testis-specific subunit of TFIIA/ALF to promote haploid cell gene expression
Abstract
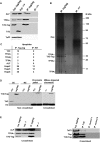
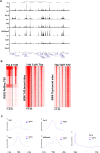
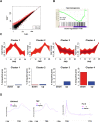
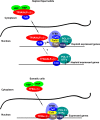
Mammalian genomes encode two genes related to the TATA-box binding protein (TBP), TBP-related factors 2 and 3 (TRF2 and TRF3). Male Trf2(-/-) mice are sterile and characterized by arrested spermatogenesis at the transition from late haploid spermatids to early elongating spermatids. Despite this characterization, the molecular function of murine Trf2 remains poorly characterized and no direct evidence exists to show that it acts as a bona fide chromatin-bound transcription factor. We show here that Trf2 forms a stable complex with TFIIA or the testis expressed paralogue ALF chaperoned in the cytoplasm by heat shock proteins. We demonstrate for the first time that Trf2 is recruited to active haploid cell promoters together with Tbp, Taf7l and RNA polymerase II. RNA-seq analysis identifies a set of genes activated in haploid spermatids during the first wave of spermatogenesis whose expression is down-regulated by Trf2 inactivation. We therefore propose that Trf2 is recruited to the preinitiation complex as a testis-specific subunit of TFIIA/ALF that cooperates with Tbp and Taf7l to promote haploid cell gene expression.
Figures


Similar articles
-
Vertebrate TBP-like protein (TLP/TRF2/TLF) stimulates TATA-less terminal deoxynucleotidyl transferase promoters in a transient reporter assay, and TFIIA-binding capacity of TLP is required for this function.Nucleic Acids Res. 2003 Apr 15;31(8):2127-33. doi: 10.1093/nar/gkg315. Nucleic Acids Res. 2003. PMID: 12682363 Free PMC article.
-
Specific interaction with transcription factor IIA and localization of the mammalian TATA-binding protein-like protein (TLP/TRF2/TLF).J Biol Chem. 2004 Feb 27;279(9):7447-55. doi: 10.1074/jbc.M305412200. Epub 2003 Oct 21. J Biol Chem. 2004. PMID: 14570910
-
Taf7l cooperates with Trf2 to regulate spermiogenesis.Proc Natl Acad Sci U S A. 2013 Oct 15;110(42):16886-91. doi: 10.1073/pnas.1317034110. Epub 2013 Sep 30. Proc Natl Acad Sci U S A. 2013. PMID: 24082143 Free PMC article.
-
Role of the TATA-box binding protein (TBP) and associated family members in transcription regulation.Gene. 2022 Jul 30;833:146581. doi: 10.1016/j.gene.2022.146581. Epub 2022 May 18. Gene. 2022. PMID: 35597524 Review.
-
TRF2: TRansForming the view of general transcription factors.Transcription. 2015;6(1):1-6. doi: 10.1080/21541264.2015.1004980. Epub 2015 Jan 14. Transcription. 2015. PMID: 25588059 Free PMC article. Review.
Cited by
-
The SAGA core module is critical during Drosophila oogenesis and is broadly recruited to promoters.PLoS Genet. 2021 Nov 22;17(11):e1009668. doi: 10.1371/journal.pgen.1009668. eCollection 2021 Nov. PLoS Genet. 2021. PMID: 34807910 Free PMC article.
-
A heterochromatin-dependent transcription machinery drives piRNA expression.Nature. 2017 Sep 7;549(7670):54-59. doi: 10.1038/nature23482. Epub 2017 Aug 23. Nature. 2017. PMID: 28847004 Free PMC article.
-
A 50-bp enhancer of the mouse acrosomal vesicle protein 1 gene activates round spermatid-specific transcription in vivo†.Biol Reprod. 2019 Oct 25;101(4):842-853. doi: 10.1093/biolre/ioz115. Biol Reprod. 2019. PMID: 31290539 Free PMC article.
-
Functionally distinct promoter classes initiate transcription via different mechanisms reflected in focused versus dispersed initiation patterns.EMBO J. 2023 May 15;42(10):e113519. doi: 10.15252/embj.2023113519. Epub 2023 Apr 4. EMBO J. 2023. PMID: 37013908 Free PMC article.
-
TATA box-binding protein-related factor 3 drives the mesendoderm specification of human embryonic stem cells by globally interacting with the TATA box of key mesendodermal genes.Stem Cell Res Ther. 2020 May 24;11(1):196. doi: 10.1186/s13287-020-01711-w. Stem Cell Res Ther. 2020. PMID: 32448362 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

