Sintokamide A Is a Novel Antagonist of Androgen Receptor That Uniquely Binds Activation Function-1 in Its Amino-terminal Domain
- PMID: 27576691
- PMCID: PMC5064002
- DOI: 10.1074/jbc.M116.734475
Sintokamide A Is a Novel Antagonist of Androgen Receptor That Uniquely Binds Activation Function-1 in Its Amino-terminal Domain
Abstract
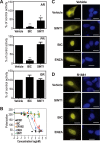
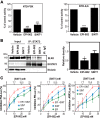
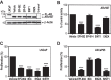
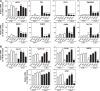
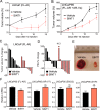
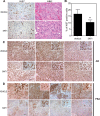
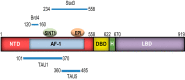
Androgen receptor (AR) is a validated drug target for all stages of prostate cancer including metastatic castration-resistant prostate cancer (CRPC). All current hormone therapies for CRPC target the C-terminal ligand-binding domain of AR and ultimately all fail with resumed AR transcriptional activity. Within the AR N-terminal domain (NTD) is activation function-1 (AF-1) that is essential for AR transcriptional activity. Inhibitors of AR AF-1 would potentially block most AR mechanisms of resistance including constitutively active AR splice variants that lack the ligand-binding domain. Here we provide evidence that sintokamide A (SINT1) binds AR AF-1 region to specifically inhibit transactivation of AR NTD. Consistent with SINT1 targeting AR AF-1, it attenuated transcriptional activities of both full-length AR and constitutively active AR splice variants, which correlated with inhibition of growth of enzalutamide-resistant prostate cancer cells expressing AR splice variants. In vivo, SINT1 caused regression of CRPC xenografts and reduced expression of prostate-specific antigen, a gene transcriptionally regulated by AR. Inhibition of AR activity by SINT1 was additive to EPI-002, a known AR AF-1 inhibitor that is in clinical trials (NCT02606123). This implies that SINT1 binds to a site on AF-1 that is unique from EPI. Consistent with this suggestion, these two compounds showed differences in blocking AR interaction with STAT3. This work provides evidence that the intrinsically disordered NTD of AR is druggable and that SINT1 analogs may provide a novel scaffold for drug development for the treatment of prostate cancer or other diseases of the AR axis.
Keywords: androgen receptor; drug development; intrinsically disordered protein; prostate cancer; steroid hormone receptor; transcription factor.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

Similar articles
-
Discovery of drugs that directly target the intrinsically disordered region of the androgen receptor.Expert Opin Drug Discov. 2020 May;15(5):551-560. doi: 10.1080/17460441.2020.1732920. Epub 2020 Feb 26. Expert Opin Drug Discov. 2020. PMID: 32100577 Free PMC article. Review.
-
An androgen receptor N-terminal domain antagonist for treating prostate cancer.J Clin Invest. 2013 Jul;123(7):2948-60. doi: 10.1172/JCI66398. Epub 2013 Jun 3. J Clin Invest. 2013. PMID: 23722902 Free PMC article.
-
Combination therapy with androgen receptor N-terminal domain antagonist EPI-7170 and enzalutamide yields synergistic activity in AR-V7-positive prostate cancer.Mol Oncol. 2020 Oct;14(10):2455-2470. doi: 10.1002/1878-0261.12770. Epub 2020 Aug 9. Mol Oncol. 2020. PMID: 32734688 Free PMC article.
-
Targeting Androgen Receptor Activation Function-1 with EPI to Overcome Resistance Mechanisms in Castration-Resistant Prostate Cancer.Clin Cancer Res. 2016 Sep 1;22(17):4466-77. doi: 10.1158/1078-0432.CCR-15-2901. Epub 2016 May 2. Clin Cancer Res. 2016. PMID: 27140928 Free PMC article.
-
Targeting the N-terminal domain of the androgen receptor: The effective approach in therapy of CRPC.Eur J Med Chem. 2023 Feb 5;247:115077. doi: 10.1016/j.ejmech.2022.115077. Epub 2022 Dec 30. Eur J Med Chem. 2023. PMID: 36587421 Review.
Cited by
-
Strategy for Tumor-Selective Disruption of Androgen Receptor Function in the Spectrum of Prostate Cancer.Clin Cancer Res. 2018 Dec 15;24(24):6509-6522. doi: 10.1158/1078-0432.CCR-18-0982. Epub 2018 Sep 5. Clin Cancer Res. 2018. PMID: 30185422 Free PMC article.
-
Non-nuclear AR Signaling in Prostate Cancer.Front Chem. 2019 Sep 26;7:651. doi: 10.3389/fchem.2019.00651. eCollection 2019. Front Chem. 2019. PMID: 31616657 Free PMC article. Review.
-
Discovery of drugs that directly target the intrinsically disordered region of the androgen receptor.Expert Opin Drug Discov. 2020 May;15(5):551-560. doi: 10.1080/17460441.2020.1732920. Epub 2020 Feb 26. Expert Opin Drug Discov. 2020. PMID: 32100577 Free PMC article. Review.
-
Differential Gene Expression Profiles between N-Terminal Domain and Ligand-Binding Domain Inhibitors of Androgen Receptor Reveal Ralaniten Induction of Metallothionein by a Mechanism Dependent on MTF1.Cancers (Basel). 2022 Jan 13;14(2):386. doi: 10.3390/cancers14020386. Cancers (Basel). 2022. PMID: 35053548 Free PMC article.
-
Pin1 inhibition improves the efficacy of ralaniten compounds that bind to the N-terminal domain of androgen receptor.Commun Biol. 2021 Mar 22;4(1):381. doi: 10.1038/s42003-021-01927-3. Commun Biol. 2021. PMID: 33753863 Free PMC article.
References
-
- Jenster G., van der Korput H. A., van Vroonhoven C., van der Kwast T. H., Trapman J., and Brinkmann A. O. (1991) Domains of the human androgen receptor involved in steroid binding, transcriptional activation, and subcellular localization. Mol. Endocrinol. 5, 1396–1404 - PubMed
-
- Simental J. A., Sar M., Lane M. V., French F. S., and Wilson E. M. (1991) Transcriptional activation and nuclear targeting signals of the human androgen receptor. J. Biol. Chem. 266, 510–518 - PubMed
-
- Jenster G., van der Korput H. A., Trapman J., and Brinkmann A. O. (1995) Identification of two transcription activation units in the N-terminal domain of the human androgen receptor. J. Biol. Chem. 270, 7341–7346 - PubMed
-
- Andersen R. J., Mawji N. R., Wang J., Wang G., Haile S., Myung J. K., Watt K., Tam T., Yang Y. C., Bañuelos C. A., Williams D. E., McEwan I. J., Wang Y., and Sadar M. D. (2010) Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor. Cancer Cell 17, 535–546 - PubMed
-
- Myung J. K., Banuelos C. A., Fernandez J. G., Mawji N. R., Wang J., Tien A. H., Yang Y. C., Tavakoli I., Haile S., Watt K., McEwan I. J., Plymate S., Andersen R. J., and Sadar M. D. (2013) An androgen receptor N-terminal domain antagonist for treating prostate cancer. J. Clin. Investig. 123, 2948–2960 - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

