Silencing vimentin expression decreases pulmonary metastases in a pre-diabetic mouse model of mammary tumor progression
- PMID: 27568979
- PMCID: PMC5332535
- DOI: 10.1038/onc.2016.305
Silencing vimentin expression decreases pulmonary metastases in a pre-diabetic mouse model of mammary tumor progression
Abstract
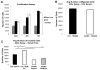
Increased breast cancer risk and mortality has been associated with obesity and type 2 diabetes (T2D). Hyperinsulinemia, a key factor in obesity, pre-diabetes and T2D, has been associated with decreased breast cancer survival. In this study, a mouse model of pre-diabetes (MKR mouse) was used to investigate the mechanisms through which endogenous hyperinsulinemia promotes mammary tumor metastases. The MKR mice developed larger primary tumors and greater number of pulmonary metastases compared with wild-type (WT) mice after injection with c-Myc/Vegf overexpressing MVT-1 cells. Analysis of the primary tumors showed significant increase in vimentin protein expression in the MKR mice compared with WT. We hypothesized that vimentin was an important mediator in the effect of hyperinsulinemia on breast cancer metastasis. Lentiviral short hairpin RNA knockdown of vimentin led to a significant decrease in invasion of the MVT-1 cells and abrogated the increase in cell invasion in response to insulin. In the pre-diabetic MKR mouse, vimentin knockdown led to a decrease in pulmonary metastases. In vitro, we found that insulin increased pAKT, prevented caspase 3 activation, and increased vimentin. Inhibiting the phosphatidylinositol 3 kinase/AKT pathway, using NVP-BKM120, increased active caspase 3 and decreased vimentin levels. This study is the first to show that vimentin has an important role in tumor metastasis in vivo in the setting of pre-diabetes and endogenous hyperinsulinemia. Vimentin targeting may be an important therapeutic strategy to reduce metastases in patients with obesity, pre-diabetes or T2D.
Conflict of interest statement
The authors disclose no potential conflicts of interest.
Figures



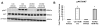



Similar articles
-
Inhibiting PI3K reduces mammary tumor growth and induces hyperglycemia in a mouse model of insulin resistance and hyperinsulinemia.Oncogene. 2012 Jul 5;31(27):3213-22. doi: 10.1038/onc.2011.495. Epub 2011 Oct 31. Oncogene. 2012. PMID: 22037215 Free PMC article.
-
EMT reversal in human cancer cells after IR knockdown in hyperinsulinemic mice.Endocr Relat Cancer. 2016 Sep;23(9):747-58. doi: 10.1530/ERC-16-0142. Epub 2016 Jul 19. Endocr Relat Cancer. 2016. PMID: 27435064 Free PMC article.
-
Hyperinsulinemia enhances c-Myc-mediated mammary tumor development and advances metastatic progression to the lung in a mouse model of type 2 diabetes.Breast Cancer Res. 2012 Jan 7;14(1):R8. doi: 10.1186/bcr3089. Breast Cancer Res. 2012. PMID: 22226054 Free PMC article.
-
Ovariectomy is associated with metabolic impairments and enhanced mammary tumor growth in MKR mice.J Endocrinol. 2015 Dec;227(3):143-151. doi: 10.1530/JOE-15-0310. Epub 2015 Sep 17. J Endocrinol. 2015. PMID: 26383532 Free PMC article.
-
Mammary tumor growth and pulmonary metastasis are enhanced in a hyperlipidemic mouse model.Oncogene. 2013 Feb 21;32(8):961-7. doi: 10.1038/onc.2012.113. Epub 2012 Apr 2. Oncogene. 2013. PMID: 22469977 Free PMC article.
Cited by
-
Elevated tumor LDLR expression accelerates LDL cholesterol-mediated breast cancer growth in mouse models of hyperlipidemia.Oncogene. 2017 Nov 16;36(46):6462-6471. doi: 10.1038/onc.2017.247. Epub 2017 Jul 31. Oncogene. 2017. PMID: 28759039 Free PMC article.
-
Vimentin is required for tumor progression and metastasis in a mouse model of non-small cell lung cancer.Oncogene. 2023 Jun;42(25):2074-2087. doi: 10.1038/s41388-023-02703-9. Epub 2023 May 9. Oncogene. 2023. PMID: 37161053 Free PMC article.
-
Activating Transcription Factor-5 Knockdown Reduces Aggressiveness of Mammary Tumor Cells and Attenuates Mammary Tumor Growth.Front Endocrinol (Lausanne). 2017 Jul 21;8:173. doi: 10.3389/fendo.2017.00173. eCollection 2017. Front Endocrinol (Lausanne). 2017. PMID: 28785242 Free PMC article.
-
Vimentin on the move: new developments in cell migration.F1000Res. 2018 Nov 15;7:F1000 Faculty Rev-1796. doi: 10.12688/f1000research.15967.1. eCollection 2018. F1000Res. 2018. PMID: 30505430 Free PMC article. Review.
-
Vimentin is a crucial target for anti-metastasis therapy of nasopharyngeal carcinoma.Mol Cell Biochem. 2018 Jan;438(1-2):47-57. doi: 10.1007/s11010-017-3112-z. Epub 2017 Jul 25. Mol Cell Biochem. 2018. PMID: 28744809
References
-
- Redaniel MT, Jeffreys M, May MT, Ben-Shlomo Y, Martin RM. Associations of type 2 diabetes and diabetes treatment with breast cancer risk and mortality: a population-based cohort study among British women. Cancer causes & control : CCC. 2012;23(11):1785–95. Epub 2012/09/14. - PubMed
-
- Yang Y, Mauldin PD, Ebeling M, Hulsey TC, Liu B, Thomas MB, et al. Effect of metabolic syndrome and its components on recurrence and survival in colon cancer patients. Cancer. 2012 Epub 2013/01/03. - PubMed
-
- Zanders MM, Boll D, van Steenbergen LN, van de Poll-Franse LV, Haak HR. Effect of diabetes on endometrial cancer recurrence and survival. Maturitas. 2013;74(1):37–43. Epub 2012/11/17. - PubMed
-
- Lawlor DA, Smith GD, Ebrahim S. Hyperinsulinaemia and increased risk of breast cancer: findings from the British Women’s Heart and Health Study. Cancer causes & control : CCC. 2004;15(3):267–75. Epub 2004/04/20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

