Progestin-only contraceptives: effects on weight
- PMID: 27567593
- PMCID: PMC5034734
- DOI: 10.1002/14651858.CD008815.pub4
Progestin-only contraceptives: effects on weight
Abstract
Background: Progestin-only contraceptives (POCs) are appropriate for many women who cannot or should not take estrogen. POCs include injectables, intrauterine contraception, implants, and oral contraceptives. Many POCs are long-acting, cost-effective methods of preventing pregnancy. However, concern about weight gain can deter the initiation of contraceptives and cause early discontinuation among users.
Objectives: The primary objective was to evaluate the association between progestin-only contraceptive use and changes in body weight.
Search methods: Until 4 August 2016, we searched MEDLINE, CENTRAL, POPLINE, LILACS, ClinicalTrials.gov, and ICTRP. For the initial review, we contacted investigators to identify other trials.
Selection criteria: We considered comparative studies that examined a POC versus another contraceptive method or no contraceptive. The primary outcome was mean change in body weight or mean change in body composition. We also considered the dichotomous outcome of loss or gain of a specified amount of weight.
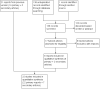
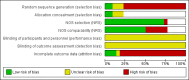
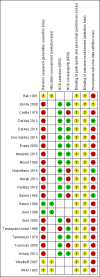
Data collection and analysis: Two authors extracted the data. Non-randomized studies (NRS) need to control for confounding factors. We used adjusted measures for the primary effects in NRS or the results of matched analysis from paired samples. If the report did not provide adjusted measures for the primary analysis, we used unadjusted outcomes. For RCTs and NRS without adjusted measures, we computed the mean difference (MD) with 95% confidence interval (CI) for continuous variables. For dichotomous outcomes, we calculated the Mantel-Haenszel odds ratio (OR) with 95% CI.
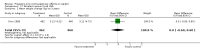
Main results: We found 22 eligible studies that included a total of 11,450 women. With 6 NRS added to this update, the review includes 17 NRS and 5 RCTs. By contraceptive method, the review has 16 studies of depot medroxyprogesterone acetate (DMPA), 4 of levonorgestrel-releasing intrauterine contraception (LNG-IUC), 5 for implants, and 2 for progestin-only pills.Comparison groups did not differ significantly for weight change or other body composition measure in 15 studies. Five studies with moderate or low quality evidence showed differences between study arms. Two studies of a six-rod implant also indicated some differences, but the evidence was low quality.Three studies showed differences for DMPA users compared with women not using a hormonal method. In a retrospective study, weight gain (kg) was greater for DMPA versus copper (Cu) IUC in years one (MD 2.28, 95% CI 1.79 to 2.77), two (MD 2.71, 95% CI 2.12 to 3.30), and three (MD 3.17, 95% CI 2.51 to 3.83). A prospective study showed adolescents using DMPA had a greater increase in body fat (%) compared with a group not using a hormonal method (MD 11.00, 95% CI 2.64 to 19.36). The DMPA group also had a greater decrease in lean body mass (%) (MD -4.00, 95% CI -6.93 to -1.07). A more recent retrospective study reported greater mean increases with use of DMPA versus Cu IUC for weight (kg) at years 1 (1.3 vs 0.2), 4 (3.5 vs 1.9), and 10 (6.6 vs 4.9).Two studies reported a greater mean increase in body fat mass (%) for POC users versus women not using a hormonal method. The method was LNG-IUC in two studies (reported means 2.5 versus -1.3; P = 0.029); (MD 1.60, 95% CI 0.45 to 2.75). One also studied a desogestrel-containing pill (MD 3.30, 95% CI 2.08 to 4.52). Both studies showed a greater decrease in lean body mass among POC users.
Authors' conclusions: We considered the overall quality of evidence to be low; more than half of the studies had low quality evidence. The main reasons for downgrading were lack of randomizations (NRS) and high loss to follow-up or early discontinuation.These 22 studies showed limited evidence of change in weight or body composition with use of POCs. Mean weight gain at 6 or 12 months was less than 2 kg (4.4 lb) for most studies. Those with multiyear data showed mean weight change was approximately twice as much at two to four years than at one year, but generally the study groups did not differ significantly. Appropriate counseling about typical weight gain may help reduce discontinuation of contraceptives due to perceptions of weight gain.
Conflict of interest statement
DECLARATIONS OF INTEREST The authors, Lopez LM, Ramesh S, Chen M, Edelman A, Otterness C, Trussell J, and Helmerhorst FM, have no conflicts of interest to declare regarding this review.
Figures

















































Update of
-
Progestin-only contraceptives: effects on weight.Cochrane Database Syst Rev. 2013 Jul 2;7(7):CD008815. doi: 10.1002/14651858.CD008815.pub3. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2016 Aug 28;(8):CD008815. doi: 10.1002/14651858.CD008815.pub4 PMID: 23821307 Free PMC article. Updated. Review.
Similar articles
-
Progestin-only contraceptives: effects on weight.Cochrane Database Syst Rev. 2013 Jul 2;7(7):CD008815. doi: 10.1002/14651858.CD008815.pub3. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2016 Aug 28;(8):CD008815. doi: 10.1002/14651858.CD008815.pub4 PMID: 23821307 Free PMC article. Updated. Review.
-
Progestin-only contraceptives: effects on weight.Cochrane Database Syst Rev. 2011 Apr 13;(4):CD008815. doi: 10.1002/14651858.CD008815.pub2. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2013 Jul 02;(7):CD008815. doi: 10.1002/14651858.CD008815.pub3 PMID: 21491411 Free PMC article. Updated. Review.
-
Combined hormonal versus nonhormonal versus progestin-only contraception in lactation.Cochrane Database Syst Rev. 2015 Mar 20;2015(3):CD003988. doi: 10.1002/14651858.CD003988.pub2. Cochrane Database Syst Rev. 2015. PMID: 25793657 Free PMC article. Review.
-
Steroidal contraceptives: effect on bone fractures in women.Cochrane Database Syst Rev. 2014 Jun 24;2014(6):CD006033. doi: 10.1002/14651858.CD006033.pub5. Cochrane Database Syst Rev. 2014. PMID: 24960023 Free PMC article. Review.
-
Steroidal contraceptives: effect on carbohydrate metabolism in women without diabetes mellitus.Cochrane Database Syst Rev. 2014 Apr 30;2014(4):CD006133. doi: 10.1002/14651858.CD006133.pub5. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2019 Nov 12;2019(11). doi: 10.1002/14651858.CD006133.pub5 PMID: 24788670 Free PMC article. Updated. Review.
Cited by
-
[Contraception in adolescents with obesity and diabetes mellitus].Probl Endokrinol (Mosk). 2022 Aug 10;68(6):137-145. doi: 10.14341/probl12760. Probl Endokrinol (Mosk). 2022. PMID: 36689719 Free PMC article. Russian.
-
Contraceptive Options Following Gestational Diabetes: Current Perspectives.Open Access J Contracept. 2019 Oct 22;10:41-53. doi: 10.2147/OAJC.S184821. eCollection 2019. Open Access J Contracept. 2019. PMID: 31749639 Free PMC article.
-
Oral contraceptive use and fracture risk-a retrospective study of 12,970 women in the UK.Osteoporos Int. 2017 Aug;28(8):2349-2355. doi: 10.1007/s00198-017-4036-x. Epub 2017 Apr 13. Osteoporos Int. 2017. PMID: 28409216
-
Towards a roadmap to advance non-hormonal contraceptive multipurpose prevention technologies: strategic insights from key stakeholders†.Biol Reprod. 2020 Aug 4;103(2):289-298. doi: 10.1093/biolre/ioaa092. Biol Reprod. 2020. PMID: 32639007 Free PMC article. Review.
-
Early discontinuation of the IMPLANON® and associated factors in Ethiopia, systematic review and meta-analysis.Heliyon. 2023 May 20;9(6):e15972. doi: 10.1016/j.heliyon.2023.e15972. eCollection 2023 Jun. Heliyon. 2023. PMID: 37251447 Free PMC article.
References
References to studies included in this review
Ball 1991 {published data only}
-
- Ball MJ, Ashwell E, Gillmer MD. Progestagen‐only oral contraceptives: comparison of the metabolic effects of levonorgestrel and norethisterone. Contraception 1991;44(3):223‐33. - PubMed
Bonny 2009 {published data only}
Castle 1978 {published data only}
-
- Castle WM, Sapire KE, Howard KA. Efficacy and acceptability of injectable medroxyprogesterone: a comparison of 3‐monthly and 6‐monthly regimens. South African Medical Journal 1978; Vol. 53, issue 21:842‐5. - PubMed
Dal'Ava 2012 {published data only}
-
- Dal'Ava N, Bahamondes L, Bahamondes MV, Oliveira Santos A, Monteiro I. Body weight and composition in users of levonorgestrel‐releasing intrauterine system. Contraception 2012;86(4):350‐3. - PubMed
Dal'Ava 2014 {published data only}
-
- Dal'Ava N, Bahamondes L, Bahamondes MV, Bottura BF, Monteiro I. Body weight and body composition of depot medroxyprogesterone acetate users. Contraception 2014;90(2):182‐7. - PubMed
Dos Santos 2014 {published data only}
-
- Cursino K, Sider M, Pavin EJ, Dos Santos PN, Bahamondes L, Zantut‐Wittmann DE, et al. Insulin resistance parameters in users of the injectable contraceptive depot medroxyprogesterone acetate during one year of use. European Journal of Contraception and Reproductive Health Care 2015;21(1):1‐8. - PubMed
-
- Dos Santos PdeNS, Modesto WO, Dal'Ava N, Bahamondes M, Pavin E, Fernades A. Body composition and weight gain in new users of the three‐monthly injectable contraceptive, depot‐medroxyprogesterone acetate, after 12 months of follow‐up. European Journal of Contraception and Reproductive Health Care 2014;19(6):432‐38. - PubMed
-
- Modesto W, Bahamondes MV, Silva dos Santos P, Fernandes A, Dal'Ava N, Bahamondes L. Exploratory study of the effect of lifestyle counselling on bone mineral density and body composition in users of the contraceptive depot‐medroxyprogesterone acetate. European Journal of Contraception and Reproductive Health Care 2014;19(4):244‐9. - PubMed
Espey 2000 {published data only}
-
- Espey E, Steinhart J, Ogburn T, Qualls C. Depo‐provera associated with weight gain in Navajo women. Contraception. 2000/12/05 2000; Vol. 62, issue 2:55‐8. - PubMed
Modesto 2015 {published data only}
-
- Modesto W, dos Santos PdeNS, Correia VM, Borges L, Bahamondes L. Weight variation in users of depot‐medroxyprogesterone acetate, the levonorgestrel‐releasing intrauterine system and a copper intrauterine device for up to ten years of use. European Journal of Contraception and Reproductive Health Care 2015;20(1):57‐63. - PubMed
Moore 1995 {published data only}
-
- Moore LL, Valuck R, McDougall C, Fink W. A comparative study of one‐year weight gain among users of medroxyprogesterone acetate, levonorgestrel implants, and oral contraceptives. Contraception. 1995/10/01 1995; Vol. 52, issue 4:215‐9. - PubMed
Napolitano 2015 {published data only}
-
- Napolitano A, Zanin R, Palma F, Romani C, Grandi G, Carlo C, et al. Body composition and resting metabolic rate of perimenopausal women using continuous progestogen contraception. European Journal of Contraception and Reproductive Health Care 2015 Aug 25 [Epub ahead of print]:1‐8. - PubMed
Nyirati 2013 {published data only}
-
- Nyirati CM, Habash DL, Shaffer E. Weight and body fat changes in postpartum depot‐medroxyprogesterone acetate users. Contraception 2013;88(1):169‐76. - PubMed
Pantoja 2010 {published data only}
-
- Pantoja M, Medeiros T, Baccarin MC, Morais S, Fernandes AM. Variation of weight among users of the contraceptive with depot‐medroxyprogesterone acetate according to body mass index in a six‐year follow‐up. Revista Brasileira de Ginecologia e Obstetricia 2009; Vol. 31, issue 8:380‐4. - PubMed
-
- Pantoja M, Medeiros T, Baccarin MC, Morais SS, Bahamondes L, Fernandes AM. Variations in body mass index of users of depot‐medroxyprogesterone acetate as a contraceptive. Contraception 2010;81(2):107‐11. - PubMed
Salem 1984 {published data only}
-
- Salem HT, Abdullah KA, Shaaban MM. Norplant and lactation. The Norplant subdermal contraceptive system. Proceedings of the Symposium on Longterm Subdermal Contraceptive Implants; 1984 February 23‐24. Assuit, Egypt: Assiut University, 1984:139‐49.
Salem 1988 {published data only}
-
- Salem HT, Salah M, Aly MY, Thabet AI, Shaaban MM, Fathalla MF. Acceptability of injectable contraceptives in Assiut, Egypt. Contraception. 1988/12/01 1988; Vol. 38, issue 6:697‐710. - PubMed
Sivin 1998 {published data only}
-
- Sivin I, Campodonico I, Kiriwat O, Holma P, Diaz S, Wan L, et al. The performance of levonorgestrel rod and Norplant contraceptive implants: a 5 year randomized study. Human Reproduction. 1999/01/14 1998; Vol. 13, issue 12:3371‐8. - PubMed
-
- Sivin I, Viegas O, Campodonico I, Diaz S, Pavez M, Wan L, et al. Clinical performance of a new two‐rod levonorgestrel contraceptive implant: a three‐year randomized study with Norplant implants as controls. Contraception 1997;55(2):73‐80. - PubMed
Sule 2005 {published data only}
-
- Sule S, Shittu O. Weight changes in clients on hormonal contraceptives in Saria, Nigeria. African Journal of Reproductive Health 2005;9(2):92‐100. - PubMed
Taneepanichskul 1998 {published data only}
-
- Taneepanichskul S, Reinprayoon D, Jaisamrarn U. Effects of DMPA on weight and blood pressure in long term acceptors. Contraception 1999;59(5):301‐3. - PubMed
-
- Taneepanichskul S, Reinprayoon D, Khaosaad P. Comparative study of weight change between long‐term DMPA and IUD acceptors. Contraception 1998;58(3):149‐51.
Tankeyoon 1976 {published data only}
-
- Tankeyoon M, Dusitsin N, Poshyachinda V, Larsson‐Cohn U. A study of glucose tolerance, serum transaminase and lipids in women using depot‐medroxyprogesterone acetate and a combination‐type oral contraceptive. Contraception 1976; Vol. 14, issue 2:199‐214. - PubMed
Tuchman 2005 {published data only}
-
- Tuchman LK, Huppert JS, Huang B, Slap GB. Adolescent use of the monthly contraceptive injection. Journal of Pediatric and Adolescent Gynecology 2005; Vol. 18, issue 4:255‐60. - PubMed
Vickery 2013 {published data only}
Westhoff 2007 {published data only}
-
- Jain J, Jakimiuk AJ, Bode FR, Ross D, Kaunitz AM. Contraceptive efficacy and safety of DMPA‐SC. Contraception 2004;70(4):269‐75. - PubMed
-
- Westhoff C, Jain JK, Milsom I, Ray A. Changes in weight with depot medroxyprogesterone acetate subcutaneous injection 104 mg/0.65 mL. Contraception 2007;75(4):261‐7. - PubMed
WHO 1983 {published data only}
-
- World Health Organization. Multinational comparative clinical trial of long‐acting injectable contraceptives: norethisterone enanthate given in two dosage regimens and depot‐medroxyprogesterone acetate. A preliminary report. Contraception. 1982/01/01 1982; Vol. 25, issue 1:1‐11. - PubMed
-
- World Health Organization. Multinational comparative clinical trial of long‐acting injectable contraceptives: norethisterone enanthate given in two dosage regimens and depot‐medroxyprogesterone acetate. Final report. Contraception. 1983/07/01 1983; Vol. 28, issue 1:1‐20. - PubMed
References to studies excluded from this review
Agoestina 1978 {published data only}
-
- Agoestina T, Biben A. Use of Depo Provera in immediate post partum and lactating period at Dr Hasan Sadikin Hospital (1978). Report on file 10.
Bahamondes 2010 {published data only}
-
- Bahamondes MV, Monteiro I, Castro S, Espejo‐Arce X, Bahamondes L. Prospective study of the forearm bone mineral density of long‐term users of the levonorgestrel‐releasing intrauterine system. Human Reproduction 2010;25(5):1158‐64. - PubMed
Barsivala 1974 {published data only}
-
- Barsivala V, Virkar K, Kulkarni RD. Thyroid functions of women taking oral contraceptives. Contraception 1974; Vol. 9, issue 3:305‐14. - PubMed
Beksinska 2010 {published data only}
Berenson 1997 {published data only}
-
- Berenson AB, Wiemann CM, Rickerr VI, McCombs SL. Contraceptive outcomes among adolescents prescribed Norplant implants versus oral contraceptives after one year of use. American Journal of Obstetrics and Gynecology 1997;176(3):586‐92. - PubMed
Berenson 2009 {published data only}
Bonny 2006 {published data only}
-
- Bonny A, Camlin K, Harvey R, Islam KM, Debanne S. Prospective analysis of weight changes in adolescent females initiating depot medroxyprogesterone acetate (DMPA), oral contraceptive pills (OC), or no hormonal contraceptive method. Journal of Adolescent Health 2003;32(2):134.
-
- Bonny AE, Ziegler J, Harvey R, Debanne SM, Secic M, Cromer BA. Weight gain in obese and nonobese adolescent girls initiating depot medroxyprogesterone, oral contraceptive pills, or no hormonal contraceptive method. Archives of Pediatrics & Adolescent Medicine. 2006/01/04 2006; Vol. 160, issue 1:40‐5. - PubMed
Bonny 2011 {published data only}
Bonny 2015 {published data only}
Casey 2013 {published data only}
-
- Casey PM, Long ME, Marnach ML, Fleming‐Harvey J, Drozdowicz LB, Weaver AL. Association of body mass index with removal of etonogestrel subdermal implant. Contraception 2013;87(3):370‐4. - PubMed
Chen 2011 {published data only (unpublished sought but not used)}
-
- Chen J, Wang XJ, Liang Y, Jin Q. [Clinic observation of a levonorgestrel‐releasing intrauterine system inserted immediately after artificial abortion]. Zhonghua yi xue za zhi 2011;91(45):3176‐8. - PubMed
Clark 2005 {published data only}
-
- Clark MK, Dillon JS, Sowers M, Nichols S. Weight, fat mass, and central distribution of fat increase when women use depot‐medroxyprogesterone acetate for contraception. International Journal of Obesity 2005; Vol. 29, issue 10:1252‐8. - PubMed
-
- Clark MK, Sowers MR, Nichols S, Levy B. Bone mineral density changes over two years in first‐time users of depot medroxyprogesterone acetate. Fertility and Sterility. 2004/12/14 2004; Vol. 82, issue 6:1580‐6. - PubMed
Costa 2012 {published data only}
-
- Costa ML, Cecatti JG, Krupa FG, Rehder PM, Sousa MH, Costa‐Paiva L. Progestin‐only contraception prevents bone loss in postpartum breastfeeding women. Contraception 2012;85(4):374‐80. - PubMed
Dahlberg 1982 {published data only}
-
- Dahlberg K. Some effects of depo‐medroxyprogesterone acetate (DMPA): observations in the nursing infant and in the long‐term user. International Journal of Gynaecology and Obstetrics 1982; Vol. 20, issue 1:43‐8. - PubMed
El Mahgoub 1980 {published data only}
-
- Mahgoub S. Body weight and cycle control of injectable contraceptives. Journal of Reproductive Medicine 1980;24(3):119‐26. - PubMed
Gallo 2016 {published data only}
Hall 1980 {published data only}
-
- Hall WD, Douglas MB, Blumenstein BA, Hatcher RA. Blood pressure and oral progestational agents. A prospective study of 119 black women. American Journal of Obstetrics and Gynecology 1980;136(3):344‐8. - PubMed
Havranek 1972 {published data only}
-
- Havranek F, Tichy M, Divila F, Hejda V. Experience with the contraceptive effect of Depo‐Provera, 300mg and 450mg administered at 6 month intervals [Zkusenosti s antikoncepcni ucinnosti Depo‐Provery v davce 300mg a 450mg podanych v intervalech 6 mesicu]. Ceskoslovenska Gynekologie 1972; Vol. 37, issue 2:95‐6. - PubMed
Hernandez‐Juarez 2014 {published data only}
Kaunitz 2009 {published data only}
-
- Kaunitz AM, Darney PD, Ross D, Wolter KD, Speroff L. Subcutaneous DMPA vs. intramuscular DMPA: a 2‐year randomized study of contraceptive efficacy and bone mineral density. Contraception 2009;80(1):7‐17. - PubMed
Mangan 2002 {published data only}
-
- Mangan SA, Larsen PG, Hudson S. Overweight teens at increased risk for weight gain while using depot medroxyprogesterone acetate. Journal of Pediatric and Adolescent Gynecology 2002; Vol. 15, issue 2:79‐82. - PubMed
Modesto 2014b {published data only}
-
- Modesto W, Bahamondes MV, Bahamondes L. A randomized clinical trial of the effect of intensive versus non‐intensive counselling on discontinuation rates due to bleeding disturbances of three long‐acting reversible contraceptives. Human Reproduction 2014;29(7):1393‐9. - PubMed
Nault 2013 {published data only}
Olsson 1988 {published data only}
-
- Olsson SE, Odlind V, Johansson ED, Sivin I. Contraception with NORPLANT implants and NORPLANT‐2 implants (two covered rods). Results from a comparative clinical study in Sweden. Contraception. 1988/01/01 1988; Vol. 37, issue 1:61‐73. - PubMed
Ortayli 2001 {published data only}
-
- Ortayli N, Bulut A, Sahin T, Sivin I. Immediate postabortal contraception with the levonorgestrel intrauterine device, Norplant, and traditional methods. Contraception 2001;63(6):309‐14. - PubMed
Risser 1999 {published data only}
-
- Risser WL, Gefter LR, Barratt MS, Risser JM. Weight change in adolescents who used hormonal contraception. Journal of Adolescent Health. 1999/07/13 1999; Vol. 24, issue 6:433‐6. - PubMed
Segall‐Gutierrez 2012 {published data only}
-
- Segall‐Gutierrez P, Xiang A H, Watanabe R M, Trigo E, Stanczyk F Z, Liu X, et al. Deterioration in cardiometabolic risk markers in obese women during depot medroxyprogesterone acetate use. Contraception 2012;85(1):36‐41. - PubMed
Veisi 2013 {published data only}
WHO 1978 {published data only}
-
- World Health Organization. Multinational comparative clinical evaluation of two long‐acting injectable contraceptive steroids: norethisterone oenanthate and medroxyprogesterone acetate. 2. Bleeding patterns and side effects. Contraception. 1978/05/01 1978; Vol. 17, issue 5:395‐406. - PubMed
Yela 2006 {published data only}
-
- Yela DA, Monteiro IM, Bahamondes LG, Castillo SD, Bahamondes MV. Weight variation in users of the levonorgestrel‐releasing intrauterine system, of the copper IUD and of medroxyprogesterone acetate in Brazil. Revista da Associacao Medica Brasileira 2006; Vol. 52, issue 1:32‐6. - PubMed
Zheng 1999 {published data only}
-
- Zheng SR, Zheng HM, Qian SZ, Sang GW, Kaper RF. A randomized multicenter study comparing the efficacy and bleeding pattern of a single‐rod (Implanon) and a six‐capsule (Norplant) hormonal contraceptive implant. Contraception. 1999/11/05 1999; Vol. 60, issue 1:1‐8. - PubMed
References to studies awaiting assessment
Madden 2014 {published data only}
-
- Madden TE. Comparison of Body Composition & Weight Change in Users of Progestin‐only Contraception During the First Year of Use (DEXA). clinicaltrials.gov/ct2/show/NCT01579773 (accessed 29 September 2015).
Schreiber 2014 {published data only}
-
- Schreiber CA. The Impact of Contraception on Postpartum Weight Loss (PPWL). clinicaltrials.gov/ct2/show/NCT01579773 (accessed 29 September 2015).
References to ongoing studies
Bonny 2016 {published data only}
-
- Bonny A. Drug Exposure and Depot Medroxyprogesterone Acetate (DMPA) in Adolescent Subjects. clinicaltrials.gov/ct2/show/NCT01461824 (accessed 29 September 2015).
Halpern 2017 {published data only}
-
- Halpern V. Pharmacodynamics and Pharmacokinetics Study of Existing DMPA Contraceptive Methods. clinicaltrials.gov/ct2/show/NCT02456584 (accessed 16 October 2016).
Additional references
ACOG 2006
-
- American College of Obstetricians and Gynecologists. ACOG technical bulletin. Use of hormonal contraception in women with coexisting medical conditions. Washington, D.C.: American College of Obstetricians and Gynecologists, 2006.
Albright 2015
-
- Albright M, Rani S, Gavagan T. HelpDesk answers: do hormonal contraceptives lead to weight gain?. Journal of Family Practice 2015;64(6):371‐2. - PubMed
Bartz 2011
-
- Bartz D, Goldberg AB. Injectable contraceptives. In: Hatcher RA, Trussell J, Nelson AL, Cates W, Kowal D, Policar MS editor(s). Contraceptive Technology. 20th Edition. New York: Ardent Media, Inc., 2011:209‐36.
Basu 2016
-
- Basu T, Bao P, Lerner A, Anderson L, Page K, Stanczyk F, et al. The effect of depo medroxyprogesterone acetate (DMPA) on cerebral food motivation centers: a pilot study using functional magnetic resonance imaging. Contraception 2016 Apr 27 [Epub ahead of print]:1‐7. - PubMed
Bender 2013
-
- Bender NM, Segall‐Gutierrez P, Najera SO, Stanczyk FZ, Montoro M, Mishell DR Jr. Effects of progestin‐only long‐acting contraception on metabolic markers in obese women. Contraception 2013;88(3):418‐25. - PubMed
Berenson 2008
Bonny 2004
-
- Bonny AE, Britto MT, Huang B, Succop P, Slap GB. Weight gain, adiposity, and eating behaviors among adolescent females on depot medroxyprogesterone acetate (DMPA). Journal of Pediatric and Adolescent Gynecology 2004;17(2):109‐15. - PubMed
Callegari 2014
-
- Callegari LS, Nelson KM, Arterburn DE, Prager SW, Schiff MA, Schwarz EB. Factors associated with lack of effective contraception among obese women in the United States. Contraception 2014;90(3):265‐71. - PubMed
CDC 2012a
-
- Centers for Disease Control and Prevention. Defining Overweight and Obesity. http://www.cdc.gov/obesity/adult/defining.html (accessed 19 December 2012).
CDC 2012b
-
- Centers for Disease Control and Prevention. Summary Chart of U.S. Medical Eligibility Criteria for Contraceptive Use. www.cdc.gov/reproductivehealth/unintendedpregnancy/usmec.htm (accessed 1 March 2016).
Curtis 2009
-
- Curtis KM, Ravi A, Gaffield ML. Progestogen‐only contraceptive use in obese women. Contraception. 2009/09/16 2009; Vol. 80, issue 4:346‐54. - PubMed
Dickerson 2013
-
- Dickerson LM, Diaz VA, Jordon J, Davis E, Chirina S, Goddard JA, et al. Satisfaction, early removal, and side effects associated with long‐acting reversible contraception. Family Medicine 2013;45(10):701‐7. - PubMed
Edelman 2014
Flegal 2000
-
- Flegal KM, Troiano RP. Changes in the distribution of body mass index of adults and children in the US population. International Journal of Obesity and Related Metabolic Disorders 2000;24(7):807‐18. - PubMed
Flegal 2012
-
- Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999‐2010. JAMA 2012;307(5):491‐7. - PubMed
Gallo 2014
GRADE 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE Handbook [updated October 2013]. http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook... (accessed 29 December 2015).
Grimes 2007
-
- Grimes DA, Lopez LM, Manion C, Schulz KF. Cochrane systematic reviews of IUD trials: lessons learned. Contraception 2007;75(6 Suppl):S55‐9. - PubMed
Grimes 2013
Guttmacher 2015
-
- Guttmacher Institute. Contraceptive Use in the United States. October 2015. www.guttmacher.org/pubs/fb_contr_use.html (accessed 2 March 2016).
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. John Wiley & Sons, Ltd, (accessed 1 October 2015).
Kavanaugh 2015
Kohn 2015
-
- Kohn JE, Lopez PM, Simons HR. Weight and body mass index among female contraceptive clients. Contraception 2015;91(6):470‐3. - PubMed
Lange 2015
Le 2009
Lopez 2013
Merki‐Feld 2015
-
- Merki‐Feld GS, Skouby S, Serfaty D, Lech M, Bitzer J, Crosignani PG, et al. European society of contraception statement on contraception in obese women. European Journal of Contraception and Reproductive Health Care 2015;20(1):19‐28. - PubMed
Ogden 2014
Paul 1997
-
- Paul C, Skegg DC, Williams S. Depot medroxyprogesterone acetate. Patterns of use and reasons for discontinuation. Contraception. 1997/12/31 1997; Vol. 56, issue 4:209‐14. - PubMed
Prentice 2006
-
- Prentice AM. The emerging epidemic of obesity in developing countries. International Journal of Epidemiology 2006;35(1):93‐9. - PubMed
Raymond 2011
-
- Raymond EG. Progestin‐only pills. In: Hatcher RA, Trussell J, Nelson AL, Cates W Jr, Kowal D, Policar MS editor(s). Contraceptive technology. 20th Edition. New York: Ardent Media, Inc., 2011:237‐47.
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robinson 2013
Silva‐Filho 2016
-
- Silva‐Filho AL, Lira J, Rocha AL, Ferreira MC, Lamaita RM, Candido EB, et al. Non‐hormonal and hormonal intrauterine contraception: survey of patients' perceptions in four Latin American countries. European Journal of Contraception and Reproductive Health Care 2016 Feb 5 [Epub ahead of print]:1‐7. - PubMed
Sivin 2003
-
- Sivin I. Risks and benefits, advantages and disadvantages of levonorgestrel‐releasing contraceptive implants. Drug Safety. 2003/03/26 2003; Vol. 26, issue 5:303‐35. - PubMed
Steenland 2013
-
- Steenland MW, Zapata LB, Brahmi D, Marchbanks PA, Curtis KM. Appropriate follow up to detect potential adverse events after initiation of select contraceptive methods: a systematic review. Contraception 2013;87(5):611‐24. - PubMed
Steward 2016
-
- Steward RG, Bateman LA, Slentz C, Stanczyk FZ, Price TM. The impact of short‐term depot‐medroxyprogesterone acetate treatment on resting metabolic rate. Contraception 2016;93(4):317‐22. - PubMed
Trussell 2011
UN 2015
-
- United Nations, Department of Economic and Social Affairs. World Contraceptive Patterns 2015. www.un.org/en/development/desa/population/publications/ (accessed 23 February 2015).
WebMD 2010
-
- WebMD. Progestin‐only hormonal methods (mini‐pills, implants, and shots). http://www.webmd.com/sex/birth‐control/progestin‐only‐hormonal‐methods‐m... (accessed 15 Jun 2010).
Wells 2014
-
- GA Wells, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 6 October 2015).
WHO 2015a
-
- World Health Organization, Department of Reproductive Healh. Medical eligibility criteria for contraceptive use. Fifth edition ‐ Executive Summary, 2015. http://www.who.int/reproductivehealth/publications/family_planning/Ex‐Su... (accessed 2 July 2015).
WHO 2015b
-
- World Health Organization, Department of Reproductive Health. Medical eligibility criteria wheel for contraceptive use. 2015. http://www.who.int/reproductivehealth/publications/family_planning/Ex‐Su... (accessed 2 July 2015).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

