Coordination between T helper cells, iNKT cells, and their follicular helper subsets in the humoral immune response against Clostridium difficile toxin B
- PMID: 27566831
- PMCID: PMC5235901
- DOI: 10.1189/jlb.4A0616-271R
Coordination between T helper cells, iNKT cells, and their follicular helper subsets in the humoral immune response against Clostridium difficile toxin B
Abstract
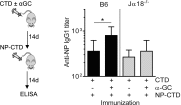
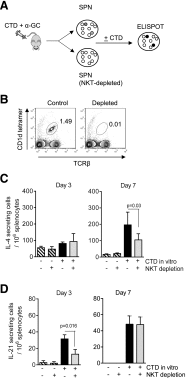
Activation of iNKT cells with the CD1d-binding glycolipid adjuvant α-galactosylceramide (α-GC) enhances humoral immunity specific for coadministered T-dependent Ag. However, the relationship between the iNKT cell and the classic T helper (Th) or T follicular helper (Tfh) function following this immunization modality remains unclear. We show that immunization with the C-terminal domain (CTD) of Clostridium difficile toxin B (TcdB), accompanied by activation of iNKT cells with α-GC, led to enhanced production of CTD-specific IgG, which was CD1d- and iNKT cell-dependent and associated with increased neutralization of active TcdB. Immunization with CTD plus α-GC followed by NP hapten-linked CTD increased NP-specific IgG1 titers in an NKT-dependent manner, suggesting that iNKT activation could enhance Th or Tfh function or that iNKT and iNKTfh cells could provide supplemental, yet independent, B cell help. Th, Tfh, iNKT, and iNKTfh cells were, therefore, examined quantitatively, phenotypically, and functionally following immunization with CTD or with CTD plus α-GC. Our results demonstrated that α-GC-activated iNKT cells had no direct effect on the numbers, phenotype, or function of Th or Tfh cells. However, CD4+ T cell-specific ablation of the Bcl6 transcription factor demonstrated that Tfh and iNKTfh cells both contributed to B cell help. This work extends our understanding of the immune response to vaccination and demonstrates an important contribution by NKTfh cells to humoral immunity.
Keywords: CD1d; T follicular helper; humoral immunity; iNKT follicular helper.
© Society for Leukocyte Biology.
Figures



Similar articles
-
Immunization-Expanded NKT Follicular Helper Cells Drive IgG1 Isotype Switch against an Exogenous T-Independent Polysaccharide but Do Not Promote Recall Responses.Immunohorizons. 2019 Mar;3(3):88-93. doi: 10.4049/immunohorizons.1800081. Immunohorizons. 2019. PMID: 31342012 Free PMC article.
-
Follicular helper NKT cells induce limited B cell responses and germinal center formation in the absence of CD4(+) T cell help.J Immunol. 2012 Apr 1;188(7):3217-22. doi: 10.4049/jimmunol.1103501. Epub 2012 Feb 29. J Immunol. 2012. PMID: 22379027 Free PMC article.
-
The CD1d-binding glycolipid alpha-galactosylceramide enhances humoral immunity to T-dependent and T-independent antigen in a CD1d-dependent manner.Immunology. 2006 Sep;119(1):116-25. doi: 10.1111/j.1365-2567.2006.02413.x. Epub 2006 Jun 22. Immunology. 2006. PMID: 16792697 Free PMC article.
-
Vaccine Designs Utilizing Invariant NKT-Licensed Antigen-Presenting Cells Provide NKT or T Cell Help for B Cell Responses.Front Immunol. 2018 Jun 4;9:1267. doi: 10.3389/fimmu.2018.01267. eCollection 2018. Front Immunol. 2018. PMID: 29915600 Free PMC article. Review.
-
The Influence of Invariant Natural Killer T Cells on Humoral Immunity to T-Dependent and -Independent Antigens.Front Immunol. 2018 Feb 22;9:305. doi: 10.3389/fimmu.2018.00305. eCollection 2018. Front Immunol. 2018. PMID: 29520280 Free PMC article. Review.
Cited by
-
Morbid Obesity Increases 30-Day Readmission and Morbidity in Clostridiodes difficile Infection.Obes Surg. 2021 May;31(5):2168-2173. doi: 10.1007/s11695-021-05245-9. Epub 2021 Feb 5. Obes Surg. 2021. PMID: 33544330
-
Host Immunity and Immunization Strategies for Clostridioides difficile Infection.Clin Microbiol Rev. 2023 Jun 21;36(2):e0015722. doi: 10.1128/cmr.00157-22. Epub 2023 May 10. Clin Microbiol Rev. 2023. PMID: 37162338 Free PMC article. Review.
-
IL-16 and BCA-1 Serum Levels Are Associated with Disease Severity of C. difficile Infection.Pathogens. 2021 May 20;10(5):631. doi: 10.3390/pathogens10050631. Pathogens. 2021. PMID: 34065379 Free PMC article.
-
A subset of follicular helper-like MAIT cells can provide B cell help and support antibody production in the mucosa.Sci Immunol. 2022 Jan 14;7(67):eabe8931. doi: 10.1126/sciimmunol.abe8931. Epub 2022 Jan 14. Sci Immunol. 2022. PMID: 35030034 Free PMC article.
-
Use of a Clostridioides difficile Murine Immunization and Challenge Model to Evaluate Single and Combination Vaccine Adjuvants Consisting of Alum and NKT Cell-Activating Ligands.Front Immunol. 2022 Jan 14;12:818734. doi: 10.3389/fimmu.2021.818734. eCollection 2021. Front Immunol. 2022. PMID: 35095921 Free PMC article.
References
-
- Gumperz J. E. (2006) The ins and outs of CD1 molecules: bringing lipids under immunological surveillance. Traffic 7, 2–13. - PubMed
-
- Brigl M., Tatituri R. V., Watts G. F., Bhowruth V., Leadbetter E. A., Barton N., Cohen N. R., Hsu F. F., Besra G. S., Brenner M. B. (2011) Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J. Exp. Med. 208, 1163–1177. - PMC - PubMed
-
- Belperron A. A., Dailey C. M., Bockenstedt L. K. (2005) Infection-induced marginal zone B cell production of Borrelia hermsii-specific antibody is impaired in the absence of CD1d. J. Immunol. 174, 5681–5686. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

